
271
4
Terrestrial and Inland
Water Systems
Coordinating Lead Authors:
Josef Settele (Germany), Robert Scholes (South Africa)
Lead Authors:
Richard A. Betts (UK), Stuart Bunn (Australia), Paul Leadley (France), Daniel Nepstad (USA),
Jonathan T. Overpeck (USA), Miguel Angel Taboada (Argentina)
Contributing Authors:
Rita Adrian (Germany), Craig Allen (USA), William Anderegg (USA), Celine Bellard (France),
Paulo Brando (Brazil), Louise P. Chini (New Zealand), Franck Courchamp (France),
Wendy Foden (South Africa), Dieter Gerten (Germany), Scott Goetz (USA), Nicola Golding (UK),
Patrick Gonzalez (USA), Ed Hawkins (UK), Thomas Hickler (Germany), George Hurtt (USA),
Charles Koven (USA), Josh Lawler (USA), Heike Lischke (Switzerland), Georgina M. Mace (UK),
Melodie McGeoch (Australia), Camille Parmesan (USA), Richard Pearson (UK),
Beatriz Rodriguez-Labajos (Spain), Carlo Rondinini (Italy), Rebecca Shaw (USA), Stephen Sitch
(UK), Klement Tockner (Germany), Piero Visconti (UK), Marten Winter (Germany)
Review Editors:
Andreas Fischlin (Switzerland), José M. Moreno (Spain), Terry Root (USA)
Volunteer Chapter Scientists:
Martin Musche (Germany), Marten Winter (Germany)
This chapter should be cited as:
Settele
, J., R. Scholes, R. Betts, S. Bunn, P. Leadley, D. Nepstad, J.T. Overpeck, and M.A. Taboada, 2014:
Terrestrial and inland water systems. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability.
Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the
Intergovernmental Panel on Climate Change [Field, C.B., V.R. Barros, D.J. Dokken, K.J. Mach,
M.D. Mastrandrea, T.E. Bilir, M. Chatterjee, K.L. Ebi, Y.O. Estrada, R.C. Genova, B. Girma, E.S. Kissel, A.N. Levy,
S. MacCracken, P.R. Mastrandrea, and L.L. White (eds.)]. Cambridge University Press, Cambridge, United
Kingdom and New York, NY, USA, pp. 271-359.

4
272
Executive Summary ........................................................................................................................................................... 274
4.1. Past Assessments ..................................................................................................................................................... 278
4.2. A Dynamic and Inclusive View of Ecosystems ........................................................................................................ 278
4.2.1. Ecosystems, Adaptation, Thresholds, and Tipping Points ................................................................................................................... 278
4.2.2. Methods and Models Used ............................................................................................................................................................... 279
4.2.3. Paleoecological Evidence .................................................................................................................................................................. 279
4.2.4. Multiple Stressors Interacting with Climate Change ......................................................................................................................... 283
4.2.4.1.Land Use and Cover Change ................................................................................................................................................ 283
Box 4-1. Future Land Use Changes .................................................................................................................................. 284
4.2.4.2.Nitrogen Deposition ............................................................................................................................................................. 285
4.2.4.3.Tropospheric Ozone .............................................................................................................................................................. 286
4.2.4.4.Rising Carbon Dioxide .......................................................................................................................................................... 287
4.2.4.5.Diffuse and Direct Radiation ................................................................................................................................................ 288
4.2.4.6.Invasive and Alien Species ................................................................................................................................................... 288
4.3. Vulnerability of Terrestrial and Freshwater Ecosystems to Climate Change .......................................................... 290
4.3.1. Changes in the Disturbance Regime ................................................................................................................................................. 290
4.3.2. Observed and Projected Change in Ecosystems ................................................................................................................................ 290
4.3.2.1.Phenology ............................................................................................................................................................................ 291
4.3.2.2.Primary Productivity ............................................................................................................................................................. 292
4.3.2.3.Biomass and Carbon Stocks ................................................................................................................................................. 293
4.3.2.4.Evapotranspiration and Water Use Efficiency ....................................................................................................................... 294
4.3.2.5.Changes in Species Range, Abundance, and Extinction ........................................................................................................ 294
4.3.3. Impacts on and Risks for Major Systems .......................................................................................................................................... 301
4.3.3.1.Forests and Woodlands ........................................................................................................................................................ 301
Box 4-2. Tree Mortality and Climate Change ................................................................................................................... 306
4.3.3.2.Dryland Ecosystems: Savannas, Shrublands, Grasslands, and Deserts .................................................................................. 308
Box 4-3. A Possible Amazon Basin Tipping Point ............................................................................................................. 309
4.3.3.3.Rivers, Lakes, Wetlands, and Peatlands ................................................................................................................................. 312
4.3.3.4.Tundra, Alpine, and Permafrost Systems ............................................................................................................................... 314
Box 4-4. Boreal–Tundra Biome Shift ................................................................................................................................ 316
4.3.3.5.Highly Human-Modified Systems ......................................................................................................................................... 317
4.3.4. Impacts on Key Ecosystem Services .................................................................................................................................................. 319
4.3.4.1.Habitat for Biodiversity ........................................................................................................................................................ 319
4.3.4.2.Timber and Pulp Production ................................................................................................................................................. 320
4.3.4.3.Biomass-Derived Energy ....................................................................................................................................................... 320
4.3.4.4.Pollination, Pest, and Disease Regulation ............................................................................................................................. 320
4.3.4.5.Moderation of Climate Change, Variability, and Extremes ................................................................................................... 321
Table of Contents

273
Terrestrial and Inland Water Systems Chapter 4
4
4.4. Adaptation and Its Limits ....................................................................................................................................... 321
4.4.1. Autonomous Adaptation by Ecosystems and Wild Organisms .......................................................................................................... 321
4.4.1.1.Phenological ........................................................................................................................................................................ 321
4.4.1.2.Evolutionary and Genetic ..................................................................................................................................................... 322
4.4.1.3.Migration of Species ............................................................................................................................................................ 324
4.4.2. Human-Assisted Adaptation ............................................................................................................................................................. 324
4.4.2.1.Reduction of Non-Climate Stresses and Restoration of Degraded Ecosystems ..................................................................... 324
4.4.2.2.The Size, Location, and Layout of Protected Areas ............................................................................................................... 324
4.4.2.3.Landscape and Watershed Management ............................................................................................................................. 324
4.4.2.4.Assisted Migration ............................................................................................................................................................... 325
4.4.2.5.Ex Situ Conservation ............................................................................................................................................................ 326
4.4.3. Consequences and Costs of Inaction and Benefits of Action ............................................................................................................ 326
4.4.4. Unintended Consequences of Adaptation and Mitigation ................................................................................................................ 327
4.5. Emerging Issues and Key Uncertainties .................................................................................................................. 328
References ......................................................................................................................................................................... 328
Frequently Asked Questions
4.1: How do land use and land cover changes cause changes in climate? .............................................................................................. 282
4.2: What are the non-greenhouse gas effects of rising carbon dioxide on ecosystems? ........................................................................ 287
4.3: Will the number of invasive alien species increase as a result of climate change? ........................................................................... 289
4.4: How does climate change contribute to species extinction? ............................................................................................................ 295
4.5: Why does it matter if ecosystems are altered by climate change? .................................................................................................... 319
4.6: Can ecosystems be managed to help them and people to adapt to climate change? ...................................................................... 325
4.7: What are the economic costs of changes in ecosystems due to climate change? ............................................................................. 326

274
Chapter 4 Terrestrial and Inland Water Systems
4
Executive Summary
The planet’s biota and ecosystem processes were strongly affected by past climate changes at rates of climate change lower
than those projected during the 21st century under high warming scenarios (e.g., Representative Concentration Pathway 8.5
(RCP8.5)) (high confidence). Most ecosystems are vulnerable to climate change even at rates of climate change projected under
low- to medium-range warming scenarios (e.g., RCP2.6 to RCP6.0).
The paleoecological record shows that global climate changes
comparable in magnitudes to those projected for the 21st century under all scenarios resulted in large-scale biome shifts and changes in
community composition; and that for rates projected under RCP6 and 8.5 were associated with species extinctions in some groups (high
confidence). {4.2.3}
Climate change is projected to be a powerful stressor on terrestrial and freshwater ecosystems in the second half of the 21st
century, especially under high-warming scenarios such as RCP6.0 and RCP8.5 (high confidence). Direct human impacts such as
land use and land use change, pollution, and water resource development will continue to dominate the threats to most
freshwater (high confidence) and terrestrial (medium confidence) ecosystems globally over the next 3 decades. Changing climate
exacerbates other impacts on biodiversity (high confidence).
Ecosystem changes resulting from climate change may not be fully apparent
for several decades, owing to long response times in ecological systems (medium confidence). Model-based projections imply that under low to
moderate warming scenarios (e.g., RCP2.6 to RCP6.0), direct land cover change will continue to dominate over (and conceal) climate-induced
change as a driver of ecosystem change at the global scale; for higher climate change scenarios, some model projections imply climate-driven
ecosystem changes sufficiently extensive to equal or exceed direct human impacts at the global scale (medium confidence). In high-altitude
and high-latitude freshwater and terrestrial ecosystems, climate changes exceeding those projected under RCP2.6 will lead to major changes in
species distributions and ecosystem function, especially in the second half of the 21st century (high confidence). {4.2.4, 4.3.2.5, 4.3.3, 4.3.3.1,
4.3.3.3, 4.4.1.1}
When terrestrial ecosystems are substantially altered (in terms of plant cover, biomass, phenology, or plant group dominance),
either through the effects of climate change or through other mechanisms such as conversion to agriculture or human settlement,
the local, regional, and global climates are also affected (high confidence).
The feedbacks between terrestrial ecosystems and climate
include, among other mechanisms, changes in surface albedo, evapotranspiration, and greenhouse gas (GHG) emissions and uptake. The physical
effects on the climate can be opposite in direction to the GHG effects, and can materially alter the net outcome of the ecosystem change on the
global climate (high confidence). The regions where the climate is affected may extend beyond the location of the ecosystem that has changed.
{4.2.4.1, 4.3.3.4}
Rising water temperatures, due to global warming, will lead to shifts in freshwater species distributions and worsen water quality
problems, especially in those systems experiencing high anthropogenic loading of nutrients (high confidence).
Climate change-
induced changes in precipitation will substantially alter ecologically important attributes of flow regimes in many rivers and wetlands and
exacerbate impacts from human water use in developed river basins (medium confidence). {4.3.3.3, Box CC-RF}
Many plant and animal species have moved their ranges, altered their abundance, and shifted their seasonal activities in response
to observed climate change over recent decades (high confidence). They are doing so now in many regions and will continue to do
so in response to projected future climate change (high confidence).
The broad patterns of species and biome shifts toward the poles and
higher in altitude in response to a warming climate are well established for periods thousands of years in the past (very high confidence). These
general patterns of range shifts have also been observed over the last few decades in some well-studied species groups such as insects and
birds and can be attributed to observed climatic changes (high confidence). Interactions between changing temperature, precipitation, and land
use can sometimes result in range shifts that are downhill or away from the poles. Certainty regarding past species movements in response to
changing climate, coupled with projections from a variety of models and studies, provides high confidence that such species movements will be
the norm with continued warming. Under all RCP climate change scenarios for the second half of the 21st century, with high confidence:
(1) community composition will change as a result of decreases in the abundances of some species and increases in others; and (2) the
seasonal activity of many species will change differentially, disrupting life cycles and interactions between species. Composition and seasonal
change will both alter ecosystem function. {4.2.1, 4.2.3, 4.3.2, 4.3.2.1, 4.3.2.5, 4.3.3, 4.4.1.1}

275
4
Terrestrial and Inland Water Systems Chapter 4
Many species will be unable to move fast enough during the 21st century to track suitable climates under mid- and high-range
rates of climate change (i.e., RCP4.5, RCP6.0, and RCP8.5 scenarios) (medium confidence).
The climate velocity (the rate of movement
of the climate across the landscape) will exceed the maximum velocity at which many groups of organisms, in many situations, can disperse or
migrate, except after mid-century in the RCP2.6 scenario. Populations of species that cannot keep up with their climate niche will find themselves
in unfavorable climates, unable to reach areas of potentially suitable climate. Species occupying extensive flat landscapes are particularly
vulnerable because they must disperse over longer distances than species in mountainous regions to keep pace with shifting climates. Species
with low dispersal capacity will also be especially vulnerable: examples include many plants (especially trees), many amphibians, and some
small mammals. For example, the maximum observed and modeled dispersal and establishment rates for mid- and late-successional tree
species are insufficient to track climate change except in mountainous areas, even at moderate projected rates of climate change. Barriers to
dispersal, such as habitat fragmentation, prior occupation of habitat by competing species, and human-made impediments such as dams on
rivers and urbanized areas on land, reduce the ability of species to migrate to more suitable climates (high confidence). Intentional and
accidental anthropogenic transport can speed dispersal. {4.3.2.5, 4.3.3.3}
Large magnitudes of climate change will reduce the populations, vigor, and viability of species with spatially restricted populations,
such as those confined to small and isolated habitats, mountaintops, or mountain streams, even if the species has the biological
capacity to move fast enough to track suitable climates (high confidence).
The adverse effects on restricted populations are modest for
low magnitudes of climate change (e.g., RCP2.6) but very severe for the highest magnitudes of projected climate change (e.g., RCP8.5).
{4.3.2.5, 4.3.3.4, 4.3.4.1}
The capacity of many species to respond to climate change will be constrained by non-climate factors (high confidence), including
but not limited to the simultaneous presence of inhospitable land uses, habitat fragmentation and loss, competition with alien species, exposure
to new pests and pathogens, nitrogen loading, and tropospheric ozone. {4.2.4.6, 4.3.3.5, Figure 4-4}
The establishment, growth, spread, and survival of populations of invasive alien species have increased (high confidence), but
the ability to attribute alien species invasion to climate change is low in most cases. Some invasive alien species have traits that favor
their survival and reproduction under changing climates. Future movement of species into areas where they were not present historically will
continue to be driven mainly by increased dispersal opportunities associated with human activities and by increased disturbances from natural
and anthropogenic events, in some cases facilitated and promoted by climate change. {4.2.4.6, Figure 4-4}
A large fraction of terrestrial and freshwater species face increased extinction risk under projected climate change during and
beyond the 21st century, especially as climate change interacts with other pressures, such as habitat modification, overexploitation,
pollution, and invasive species (high confidence).
The extinction risk is increased under all RCP scenarios, and the risk increases with both
the magnitude and rate of climate change. While there is medium confidence that recent warming contributed to the extinction of some species
of Central American amphibians, there is generally very low confidence that observed species extinctions can be attributed to recent climate
change. Models project that the risk of species extinctions will increase in the future owing to climate change, but there is low agreement
concerning the fraction of species at increased risk, the regional and taxonomic focus for such extinctions and the time frame over which
extinctions could occur. Modeling studies and syntheses since the AR4 broadly confirm that a large proportion of species are projected to be at
increased risk of extinction at all but the lowest levels of climate warming (RCP2.6). Some aspects leading to uncertainty in the quantitative
projections of extinction risks were not taken into account in previous models; as more realistic details are included, it has been shown that the
extinction risks may be either under- or overestimated when based on simpler models. {4.3.2.5}
Terrestrial and freshwater ecosystems have sequestered about a quarter of the carbon dioxide (CO
2
) emitted to the atmosphere
by human activities in the past 3 decades (high confidence).
The net fluxes out of the atmosphere and into plant biomass and soils show
large year-to-year variability; as a result there is low confidence in the ability to determine whether the net rate at which carbon has been
taken up by terrestrial ecosystems at the global scale has changed between the decades 1991–2000 and 2001–2010. There is high confidence
that the factors causing the current increase in land carbon include the positive effects of rising CO
2
on plant productivity, a warming climate,
nitrogen deposition, and recovery from past disturbances, but low confidence regarding the relative contribution by each of these and other
factors. {4.2.4.1, 4.2.4.2, 4.2.4.4, 4.3.2.2, 4.3.2.3, WGI AR5 6.3.1, 6.3.2.6}

276
Chapter 4 Terrestrial and Inland Water Systems
4
The natural carbon sink provided by terrestrial ecosystems is partially offset at the decadal time scale by carbon released
through the conversion of natural ecosystems (principally forests) to farm and grazing land and through ecosystem degradation
(high confidence). Carbon stored in the terrestrial biosphere is vulnerable to loss back to the atmosphere as a result of the direct
and indirect effects of climate change, deforestation, and degradation (high confidence). The net transfer of CO
2
from the
atmosphere to the land is projected to weaken during the 21st century (medium confidence). The direct effects of climate change on
stored terrestrial carbon include high temperatures, drought, and windstorms; indirect effects include increased risk of fires and pest and disease
outbreaks. Experiments and modeling studies provide medium confidence that increases in CO
2
up to about 600 ppm will continue to enhance
photosynthesis and plant water use efficiency, but at a diminishing rate; and high confidence that low availability of nutrients, particularly
nitrogen, will limit the response of many natural ecosystems to rising CO
2
. There is medium confidence that other factors associated with
global change, including high temperatures, rising ozone concentrations, and in some places drought, decrease plant productivity by amounts
comparable in magnitude to the enhancement by rising CO
2
. There are few field-scale experiments on ecosystems at the highest CO
2
concentrations projected by RCP8.5 for late in the century, and none of these include the effects of other potential confounding factors.
{4.2.4, 4.2.4.1, 4.2.4.2, 4.2.4.3, 4.2.4.4, 4.3.2.2, 4.3.3.1, Box 4-3, Box CC-VW, WGI AR5 6.4.3.3}
Increases in the frequency or intensity of ecosystem disturbances such as droughts, wind storms, fires, and pest outbreaks have
been detected in many parts of the world and in some cases are attributed to climate change (medium confidence). Changes in
the ecosystem disturbance regime beyond the range of natural variability will alter the structure, composition, and functioning
of ecosystems (high confidence).
Ecological theory and experimentation predict that ecological change resulting from altered disturbance
regimes will be manifested as relatively abrupt and spatially patchy transitions in ecosystem structure, composition, and function, rather than
gradual and spatially uniform shifts in location or abundance of species (medium confidence). {4.2.4.6, 4.3.3, 4.3.2.5, Box 4-3, Box 4-4,
Figure 4-10}
Increased tree death has been observed in many places worldwide, and in some regions has been attributed to climate change
(high confidence). In some places it is sufficiently intense and widespread as to result in forest dieback (low confidence). Forest
dieback is a major environmental risk, with potentially large impacts on climate, biodiversity, wood production, water quality, amenity, and
economic activity. In detailed regional studies in western and boreal North America, the tree mortality observed over the past few decades has
been attributed to the effects of high temperatures and drought, or to changes in the distribution and abundance of insect pests and
pathogens related, in part, to warming (high confidence). Tree mortality and associated forest dieback will become apparent in many regions
sooner than previously anticipated (medium confidence). Earlier projections of increased tree growth and enhanced forest carbon sequestration
due to increased growing season duration, rising CO
2
concentration, and atmospheric nitrogen deposition must be balanced by observations
and projections of increasing tree mortality and forest loss due to fires and pest attacks. The consequences for the provision of timber and other
wood products are projected to be highly variable between regions and products, depending on the balance of the positive versus negative
effects of global change. {4.3.2, 4.3.3.1, 4.3.3.4, 4.3.3.5, 4.3.4, 4.3.4.2, Box 4-2, Box 4-3}
There is a high risk that the large magnitudes and high rates of climate change associated with low-mitigation climate scenarios
(RCP4.5 and higher) will result within this century in abrupt and irreversible regional-scale change in the composition, structure,
and function of terrestrial and freshwater ecosystems, for example in the Amazon (low confidence) and Arctic (medium confidence),
leading to substantial additional climate change.
There are plausible mechanisms, supported by experimental evidence, observations, and
model results, for the existence of ecosystem tipping points in both boreal-tundra Arctic systems and the rainforests of the Amazon basin.
Continued climate change will transform the species composition, land cover, drainage, and permafrost extent of the boreal-tundra system,
leading to decreased albedo and the release of GHGs (medium confidence). Adaptation measures will be unable to prevent substantial change
in the boreal-Arctic system (high confidence). Climate change alone is not projected to lead to abrupt widespread loss of forest cover in the
Amazon during this century a (medium confidence), but a projected increase in severe drought episodes, together with land use change and
forest fire, would cause much of the Amazon forest to transform to less dense, drought- and fire-adapted ecosystems, and in doing so put a
large stock of biodiversity at elevated risk, while decreasing net carbon uptake from the atmosphere (low confidence). Large reductions in
deforestation, as well as wider application of effective wildfire management, lower the risk of abrupt change in the Amazon, as well as the
impacts of that change (medium confidence). {4.2.4.1, 4.3.3.1.1, 4.3.3.1.3, 4.3.3.4, Figure 4-8, Box 4-3, Box 4-4}

277
Terrestrial and Inland Water Systems Chapter 4
4
Management actions can reduce, but not eliminate, the risk of impacts to terrestrial and freshwater ecosystems due to climate
change, as well as increase the inherent capacity of ecosystems and their species to adapt to a changing climate (high confidence).
The capacity for natural adaptation by ecosystems and their constituent organisms is substantial, but for many ecosystems and species it will
be insufficient to cope with projected rates and magnitudes of climate change in the 21st century without substantial loss of species and
ecosystem services, under medium-range warming (e.g., RCP6.0) or high-range warming scenarios (e.g., RCP8.5) (medium confidence). The
capacity for ecosystems to adapt to climate change can be increased by reducing the other stresses operating on them; reducing the rate and
magnitude of climate change; reducing habitat fragmentation and increasing connectivity; maintaining a large pool of genetic diversity and
functional evolutionary processes; assisted translocation of slow moving organisms or those whose migration is impeded, along with the
species on which they depend; and manipulation of disturbance regimes to keep them within the ranges necessary for species persistence and
sustained ecosystem functioning. {4.4, 4.4.1, 4.4.2}
Adaptation responses to climate change in the urban and agricultural sectors can have unintended negative outcomes for
terrestrial and freshwater ecosystems (medium confidence). For example, adaptation responses to counter increased variability of water
supply, such as building more and larger impoundments and increased water extraction, will in many cases worsen the direct effects of climate
change in freshwater ecosystems. {4.3.3.3, 4.3.4.6}
Widespread transformation of terrestrial ecosystems in order to mitigate climate change, such as carbon sequestration through
planting fast-growing tree species into ecosystems where they did not previously occur, or the conversion of previously
uncultivated or non-degraded land to bioenergy plantations, will lead to negative impacts on ecosystems and biodiversity (high
confidence).
For example, the land use scenario accompanying the mitigation scenario RCP2.6 features a large expansion of biofuel production,
displacing natural forest cover. {4.2.4.1, 4.4.4}

278
Chapter 4 Terrestrial and Inland Water Systems
4
4.1. Past Assessments
The topics assessed in this chapter were last assessed by the IPCC in
2007, principally in WGII AR4 Chapters 3 (Kundzewicz et al., 2007) and
4
(Fischlin et al., 2007), but also in WGII AR4 Sections 1.3.4 and 1.3.5
(Rosenzweig et al., 2007). The WGII AR4 SPM stated “Observational
evidence from all continents and most oceans shows that many natural
systems are being affected by regional climate changes, particularly
temperature increases,” though they noted that documentation of
observed changes in tropical regions and the Southern Hemisphere was
sparse (Rosenzweig et al., 2007). Fischlin et al. (2007) found that 20 to
30% of the plant and animal species that had been assessed to that time
were considered to be at increased risk of extinction if the global average
temperature increase exceeds 2°C to 3°C above the preindustrial level
with medium confidence, and that substantial changes in structure and
functioning of terrestrial, marine, and other aquatic ecosystems are very
likely under that degree of warming and associated atmospheric CO
2
concentration. No time scale was associated with these findings. The
carbon stocks in terrestrial ecosystems were considered to be at high
risk from climate change and land use change. The report warned that
the capacity of ecosystems to adapt naturally to the combined effect of
climate change and other stressors is likely to be exceeded if greenhouse
gas (GHG) emission continued at or above the then-current rate.
4.2. A Dynamic and Inclusive View
of Ecosystems
There are three aspects of the contemporary scientific view of ecosystems
that are important to know for policy purposes. First, ecosystems usually
have imprecise and variable boundaries. They span a wide range of
spatial scales, nested within one another, from the whole biosphere,
down through its major ecosystem types (biomes), to local and possibly
short-lived associations of organisms. Second, the human influence on
ecosystems is globally pervasive. Humans are regarded as an integral,
rather than separate, part of social-ecological systems (Gunderson and
Holling, 2001; Berkes et al., 2003). Ecosystems are connected across
boundaries through the movement of energy, materials, and organisms,
and subsidies between terrestrial and freshwater systems are known
to be particularly important (Polis et al., 1997; Loreau et al., 2003). As
a consequence, human activities in terrestrial systems can significantly
impact freshwater ecosystems and their biota (Allan, 2004). The dynamics
of socio-ecological systems are governed not only by biophysical
processes such as energy flows, material cycles, competition, and
predation, but also by social processes such as economics, politics,
culture, and individual preferences (Walker and Salt, 2006). Third,
ecologists do not view ecosystems as necessarily inherently static and
at equilibrium in the absence of a human disturbance (Hastings, 2004).
Ecosystems vary over time and space in the relative magnitude of their
components and fluxes, even under a constant environment, owing to
internal dynamics (Scheffer, 2009). Furthermore, attempts to restrict
this intrinsic variation—or that resulting from externally generated
disturbances—are frequently futile, and may damage the capacity of
the ecosystem to adapt to a changing environment (Folke et al.,
2004). This contrasts with the popular view that ecosystems exhibit a
“balance of Nature” and benefit from being completely protected from
disturbance.
4.2.1. Ecosystems, Adaptation, Thresholds,
and Tipping Points
The term “adaptation” has different meanings in climate policy, ecology,
and evolutionary biology. In climate policy (see Glossary) it implies
human actions intended to reduce negative outcomes. In ecology,
ecosystems are said to be adaptive because their composition or function
can change in response to a changing environment, without necessarily
involving deliberate human actions (see Section 4.4.1). In evolutionary
biology, adaptation means a change in the genetic properties of a
population of individuals as a result of natural selection (Section 4.4.1.2),
a possibility seen since the Fourth Assessment Report as increasingly
relevant to climate change.
The notion of thresholds has become a prominent ecological and political
concern (Knapp, A.K. et al., 2008; Lenton et al., 2008; Leadley et al.,
2010). To avoid policy confusion, three types of threshold need to be
distinguished. The first reflects a human preference that the ecosystem
stays within certain bounds, such as above a certain forest cover. These
can be, by definition, negotiated. The second type reflects fundamental
biological or physical properties, for instance the temperature at which
frozen soils thaw (see Box 4-4) or the physiological tolerance limits of
species. The third type is caused by system dynamics: the point at which
the net effect of all the positive and negative feedback loops regulating
the system is sufficiently large and positive that a small transgression
becomes sufficiently amplified to lead to a change in ecosystem state
called a regime shift (Lenton et al., 2008). The new state exhibits different
dynamics, mean composition, sensitivity to environmental drivers, and
flows of ecosystem services relative to the prior state. This type of
threshold is called a “tipping point” (defined in the Glossary as a level
of change in system properties beyond which a system reorganizes,
often abruptly, and persists in its new state even if the drivers of the
change are abated ) and is important in the context of climate change
because its onset may be abrupt, hard to predict precisely, and effectively
irreversible (Scheffer et al., 2009; Leadley et al., 2010; Barnosky et al.,
2012; Brook et al., 2013; Hughes et al., 2013). Many examples of tipping
points have now been identified (Scheffer, 2009). Regional-scale
ecosystem tipping points have not occurred in the recent past, but there
is good evidence for tipping points in the distant past (Section 4.2.3)
and there is concern that they could occur in the near future (see Boxes
4-3 and 4-4).
The early detection and prediction of ecosystem thresholds, particularly
tipping points, is an area of active research. There are indications (Scheffer,
2009) that an increase in ecosystem variability signals the impending
approach of a threshold. In practice, such signals may not be detectable
against background noise and uncertainty until the threshold is crossed
(Biggs et al., 2009). The dynamics of ecosystems are complex and our
present level of knowledge is inadequate to predict all ecosystem
outcomes with confidence, even if the future climate were precisely
known.
Field observations over the past century in numerous locations in boreal,
temperate, and tropical ecosystems have detected biome shifts, the
replacement at a location of one suite of species by another (high
confidence). The effect is usually of biomes moving upward in elevation
and to higher latitudes (Gonzalez et al., 2010; see Figure 4-1). These shifts
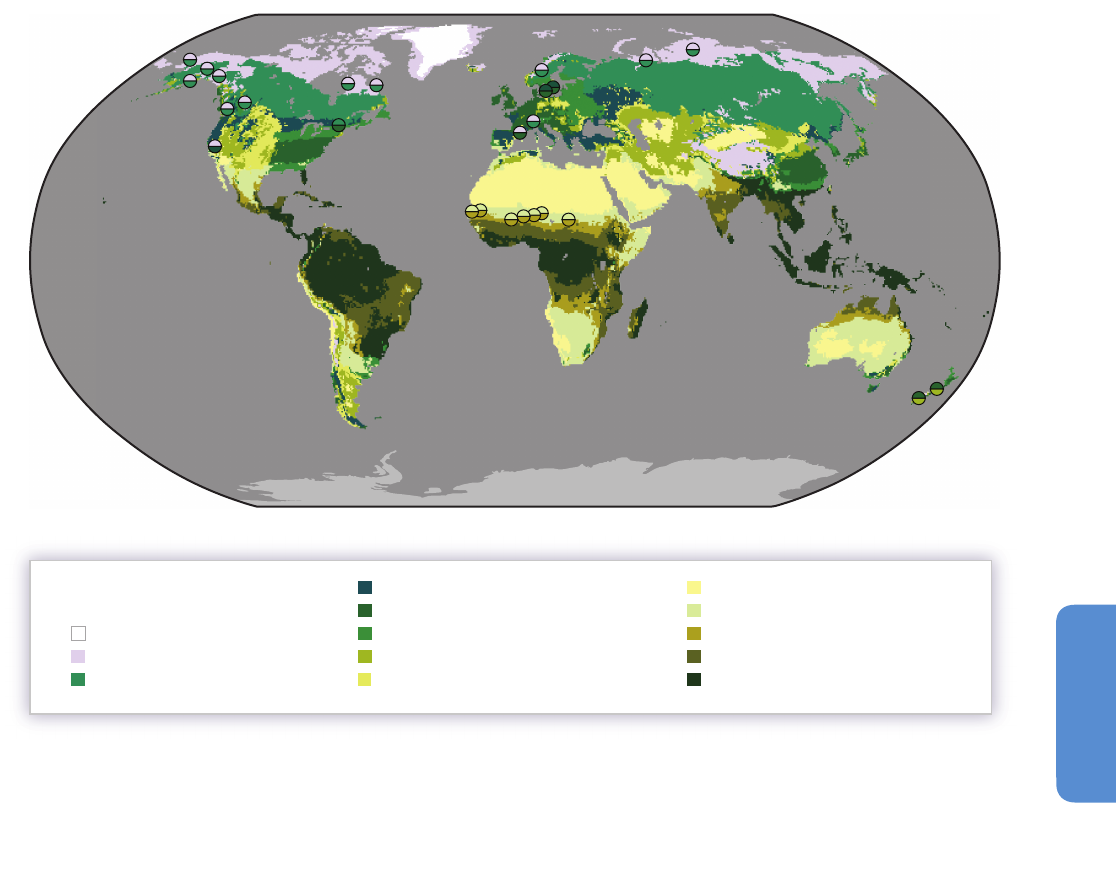
279
Terrestrial and Inland Water Systems Chapter 4
4
have often been attributed to anthropogenic climate change, as biome
distribution is known to broadly reflect climate zones, and the shifts have
been observed in areas without major human disturbance (medium
confidence; see Table 4-1). Projections of future vegetation distribution
under climate change indicate that many biomes could shift substantially,
including in areas where ecosystems are largely undisturbed by direct
human land use (Figure 4-2). The extent of the shift increases with
increasing global mean warming, without a sudden threshold (Scholze
et al., 2006; Pereira et al., 2010; Rehfeldt et al., 2012).
4.2.2. Methods and Models Used
Analysis of the current and past impacts of climate change on terrestrial
and freshwater ecosystems and their projection into the future relies
on three general approaches: inference from analogous situations in
the past or elsewhere in the present; manipulative experimentation,
deliberately altering one of a few factors at a time; and models with a
mechanistic or statistical basis. Studies of the relatively distant past
are discussed in depth in Section 4.2.3. Inferences from present spatial
patterns in relation to climate is at the core of climate envelope niche
modeling, a well-established but limited statistical technique for making
projections of the future distribution under equilibrium conditions (Elith
and Leathwick, 2009). Representing the rate of change during the non-
equilibrium conditions that will prevail over the next century requires a
more mechanistic approach, of which there are some examples (e.g.,
Keith et al., 2008; Kearney and Porter, 2009). Changes in ecosystem
function are usually determined by experimentation (see examples in
Section 4.3.3) and are modeled using mechanistic models, in many
cases with relatively high uncertainty (Seppelt et al., 2011).
4.2.3. Paleoecological Evidence
Paleoclimatic observations and modeling indicate that the Earth’s climate
has always changed on a wide range of time scales. In many cases,
particularly over the last million years, it has changed in ways that are
well understood in terms of both patterns and causes (Jansen et al.,
2007; see WGI AR5 Chapter 5). Paleoecological records demonstrate with
high confidence that the planet’s biota (both terrestrial and aquatic),
DE: Desert
RW: Tropical woodland
RD: Tropical deciduous broadleaf forest
Biomes
IC: Ice
BC: Boreal conifer forest
UA: Tundra and alpine
TC: Temperate conifer forest
TB: Temperate broadleaf forest
TM: Temperate mixed forest
TS: Temperate shrubland
TG: Temperate grassland
RG: Tropical grassland
RE: Tropical evergreen broadleaf forest
1-22: See Table 4-1
1
2
3
4
7
9
21
6
5
13
18
12
20
17
19
22
8
15
14
Figure 4-1 | Locations of observed biome shifts during the 20th century, listed in Table 4-1, derived from Gonzalez et al. (2010). The color of each semicircle indicates the
retracting biome (top for North America, Europe, Asia; bottom for Africa and New Zealand) and the expanding biome (bottom for North America, Europe, Asia; top for Africa and
New Zealand), according to published field observations. Biomes, from poles to equator: ice (IC), tundra and alpine (UA), boreal conifer forest (BC), temperate conifer forest (TC),
temperate broadleaf forest (TB), temperate mixed forest (TM), temperate shrubland (TS), temperate grassland (TG), desert (DE), tropical grassland (RG), tropical woodland (RW),
tropical deciduous broadleaf forest (RD), tropical evergreen broadleaf forest (RE). The background is the potential biome according to the MC1 dynamic global vegetation model
under the 1961–1990 climate. No shift was observed on locations 10, 11, 16, and 23 (see Table 4-1).
280
Chapter 4 Terrestrial and Inland Water Systems
4
carbon cycle, and associated feedbacks and services have responded to
this climatic change, particularly when the climatic change was as large
as that projected during the 21st century under mid- to high-end radiative
forcing pathways (e.g., MacDonald et al., 2008; Claussen, 2009; Arneth
et al., 2010; Dawson et al., 2011; Willis and MacDonald, 2011). Excellent
examples of past large climate change events that drove large ecological
change, as well as recovery periods in excess of a million years, include
the events that led to the Earth’s five mass extinctions in the distant past
(i.e., during the Ordovician, about 443 Ma, the Devonian, about 359 Ma,
the Permian, about 251 Ma, the Triassic, about 200 Ma, and the
Cretaceous, about 65 Ma; Barnosky et al., 2011). Major ecological
change was also driven by climate change during the Paleocene-Eocene
Thermal Maximum (PETM, 56 Ma; Wing et al., 2005; Jaramillo et al., 2010;
Wing and Currano, 2013), the early Eocene Climatic Optimum (EECO, 53
to 50 Ma; Woodburne et al., 2009), the Pliocene (5.3 to 2.6 Ma; Haywood
and Valdes, 2006; Haywood et al., 2011), and the Last Glacial Maximum
(LGM) to Holocene transition between 21 and 6 ka (MacDonald et al.,
2008; Clark et al., 2009; Gill et al., 2009; Williams, J.W. et al., 2010;
Prentice et al., 2011; Daniau et al., 2012). The paleoecological record thus
provides high confidence that large global climate change, comparable
in magnitude to that projected for the 21st century, can result in large
ecological changes, including large-scale biome shifts, reshuffling of
communities, and species extinctions.
Rapid, regional warming before and after the Younger Dryas cooling
event (11.7 to 12.9 ka) provides a relatively recent analogy for climate
change at a rate approaching, for many regions, that projected for the
21st century for all Representative Concentration Pathways (RCPs; Alley
et al., 2003; Steffensen et al., 2008). Ecosystems and species responded
rapidly during the Younger Dryas by shifting distributions and abundances,
and there were some notable large animal extinctions, probably
exacerbated by human activities (Gill et al., 2009; Dawson et al., 2011).
In some regions, species became locally or regionally extinct (extirpated),
but there is no evidence for climate-driven global-scale extinctions
during this period (Botkin et al., 2007; Willis, K.J. et al., 2010). However,
the Younger Dryas climate changes differ from those projected for the
future because they were regional rather than global; may have only
regionally exceeded rates of warming projected for the future; and
started from a baseline substantially colder than present (Alley et al.,
2003). The mid-Holocene, about 6 ka, provides a very recent example
of the effects of modest climate change. Regional mean warming during
this period (mean annual temperature about 0.5°C to 1.0°C above
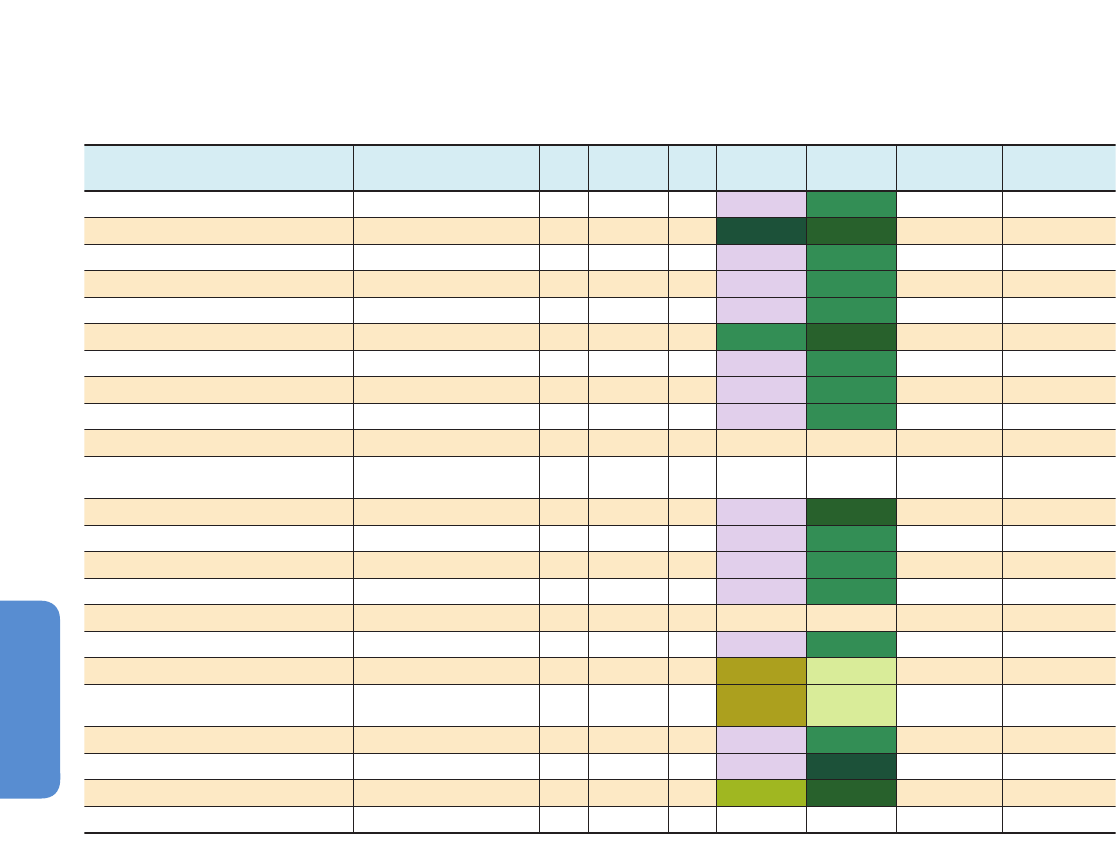
Location Reference Plots
Time
period
Shift
type
Retracting
biome
Expanding
biome
Temp. change
(ºC century
–
1
)
Precip. change
(% century
–
1
)
1. Alaska Range, Alaska, USA Lloyd and Fastie (2003) 18 1800 – 2000 L UA BC 1.1* 3
2
. Baltic Coast, Sweden Walther et al. (2005) 71944 – 2003 L
T
C TB
0
.6* 8
3
. Becca di Viou, Italy Leonelli et al. (2011) 11700 – 2008 E UA
B
C
0
.9* – 6
4. Garibaldi, British Columbia, Canada Brink (1959) 1 1860 – 1959 E UA
BC 0.7* 16*
5
. Goulet Sector, Québec, Canada Payette and Filion (1985) 21880 – 1980 E UA
B
C
1
.4* 19*
6. Green Mountains, Vermont, USA Beckage et al. (2008) 33 1962 – 2005 E
BC TB 1.6* 6
7
. Jasper, Alberta, Canada Luckman and Kavanagh (2000) 1 1700 – 1994 E UA
B
C
0
.6 21*
8. Kenai Mountains, Alaska, USA Dial et al. (2007) 31951–1996E UA
BC 0.7 6
9. Kluane Range, Yukon, Canada Danby and Hik (2007) 2 1800 – 2000 E UA
BC 0.7 5
1
0. Low Peninsula, Québec, Canada Payette and Filion (1985) 11750 – 1980 N— — 1.4* 19*
11. Mackenzie Mountains, Northwest
T
erritories, Canada
Szeicz and Macdonald (1995) 13 1700 – 1990 N— — 1.4* 3
12. Montseny Mountains, Catalonia, Spain Peñuelas and Boada (2003) 50 1945 – 2001 E UA
TB 1.2* – 3
1
3. Napaktok Bay, Labrador, Canada Payette (2007) 21750 – 2000 L UA
B
C
1
.1* 5
14. Noatak, Alaska, USA Suarez et al. (1999) 18 1700 – 1990 L UA
BC 0.6 19*
15. Putorana Mountains, Russian Federation Kirdyanov et al. (2012) 10 1500 – 2000 E UA
BC 0.3 10
16. Rahu Saddle, New Zealand Cullen et al. (2001) 71700 – 2000 N— — 0.6* 3
17. Rai-Iz, Urals, Russian Federation Devi et al. (2008) 144 1700 – 2002 E UA
BC 0.3 35*
18. Sahel, Sudan, Guinea zones; Senegal Gonzalez (2001) 135 1945 – 1993 L RW RG 0.4* – 48*
19. Sahel, Burkina Faso, Chad, Mali, Mauritania,
Niger
Gonzalez et al. (2012) 14 1960 – 2000 L RW RG – 0.01* to 0.8* – 31* to 9
20. Scandes, Sweden Kullman and Öberg (2009) 123 1915 – 2007 E UA
BC 0.8* 25*
21. Sierra Nevada, California, USA Millar et al. (2004) 10 1880 – 2002 E UA
TC – 0.1 21*
22. South Island, New Zealand Wardle and Coleman (1992) 22 1980 – 1990 E TS
TB 0.6* 3
23. Yambarran, Northern Territory, Australia Sharp and Bowman (2004) 33 1948 – 2000 N— — – 0.06 35*
Table 4-1 | Biome shifts of the 20th century from published fi eld research that examined trends over periods >30years for biomes in areas where climate (rather than land use
change or other factors) predominantly infl uenced vegetation, derived from a systematic analysis of published studies (Gonzalez et al., 2010). Pre-AR4 publications are included
to provide a comprehensive review. Shift type: elevational (E), latitudinal (L), examined but not detected (N). The biome abbreviations match those in Figure 4-1. Rate of change
in temperature (Temp.) and fractional rate of change in precipitation (Precip.) are derived from linear least squares regression of 1901– 2002 data (Mitchell and Jones, 2005;
Gonzalez et al., 2010). The table provides general regional climate trends at 50 km spatial resolution because the references do not give uniform site-specifi c climate data to
compare across locations. The regional trends are consistent with local trends reported in each reference. *Rate signifi cant at P ≤ 0.05.
281
Terrestrial and Inland Water Systems Chapter 4
4
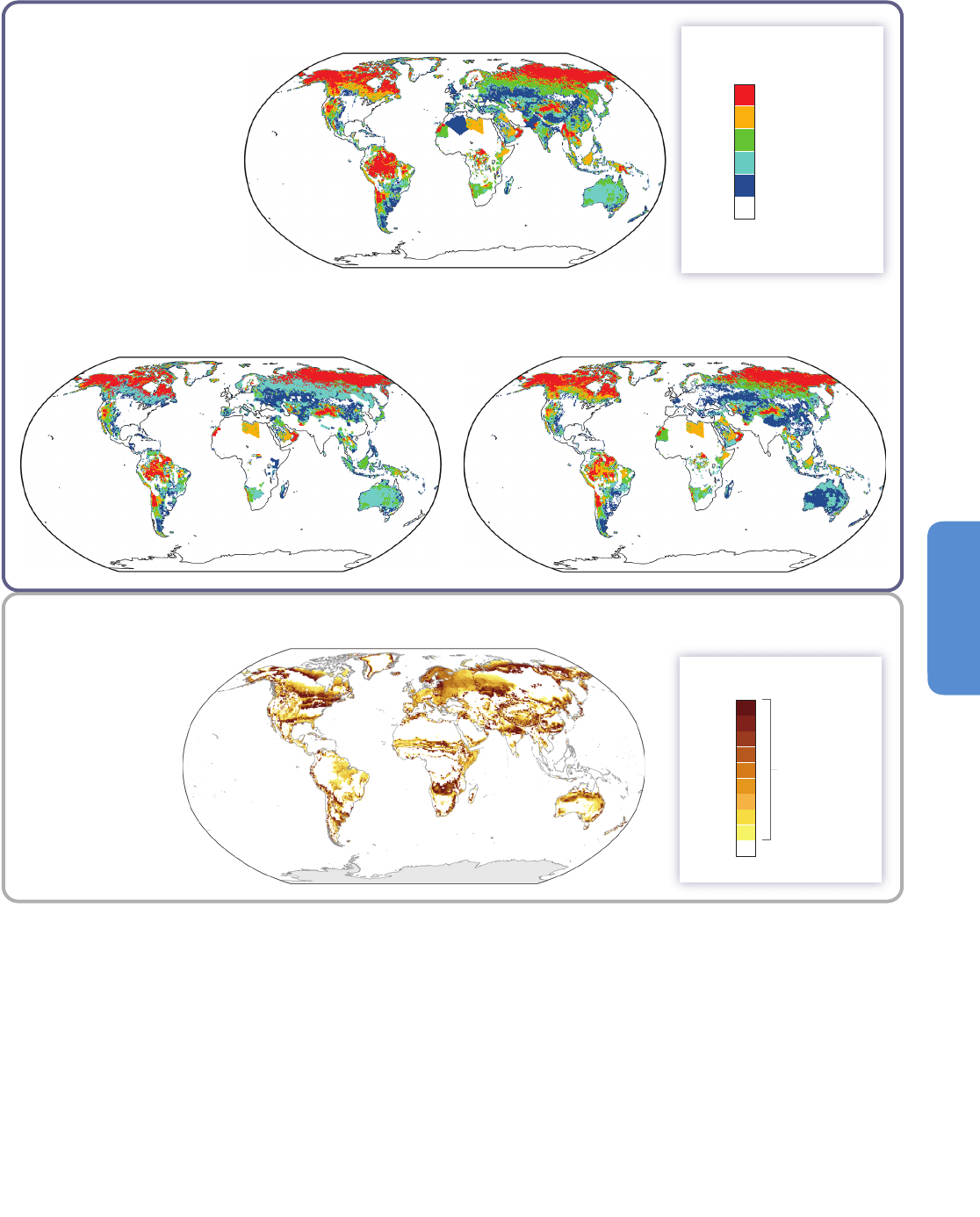
(d) Model agreement on climate change-driven biome shift between 1990 and 2100
RCP2.6 land use scenario (IMAGE model)
(a)
(c) RCP6.0 land use scenario (AIM model)
Projected primary vegetation cover in 2100
Primary vegetation cover in 2005
Percent of model agreement
P
ercent of primary vegetation*
c
over in grid cell
(b)
Comparison of panels (a), (b) and (c)
shows the effect of direct
human-induced vegetation change
through land use, without the effects
of climate change
biome shift is
projected to occur
due to climate
change
=
previously undisturbed
by human activities
no primary vegetation
*
Primary
vegetation
100
0
50
0
20
40
60
80
100
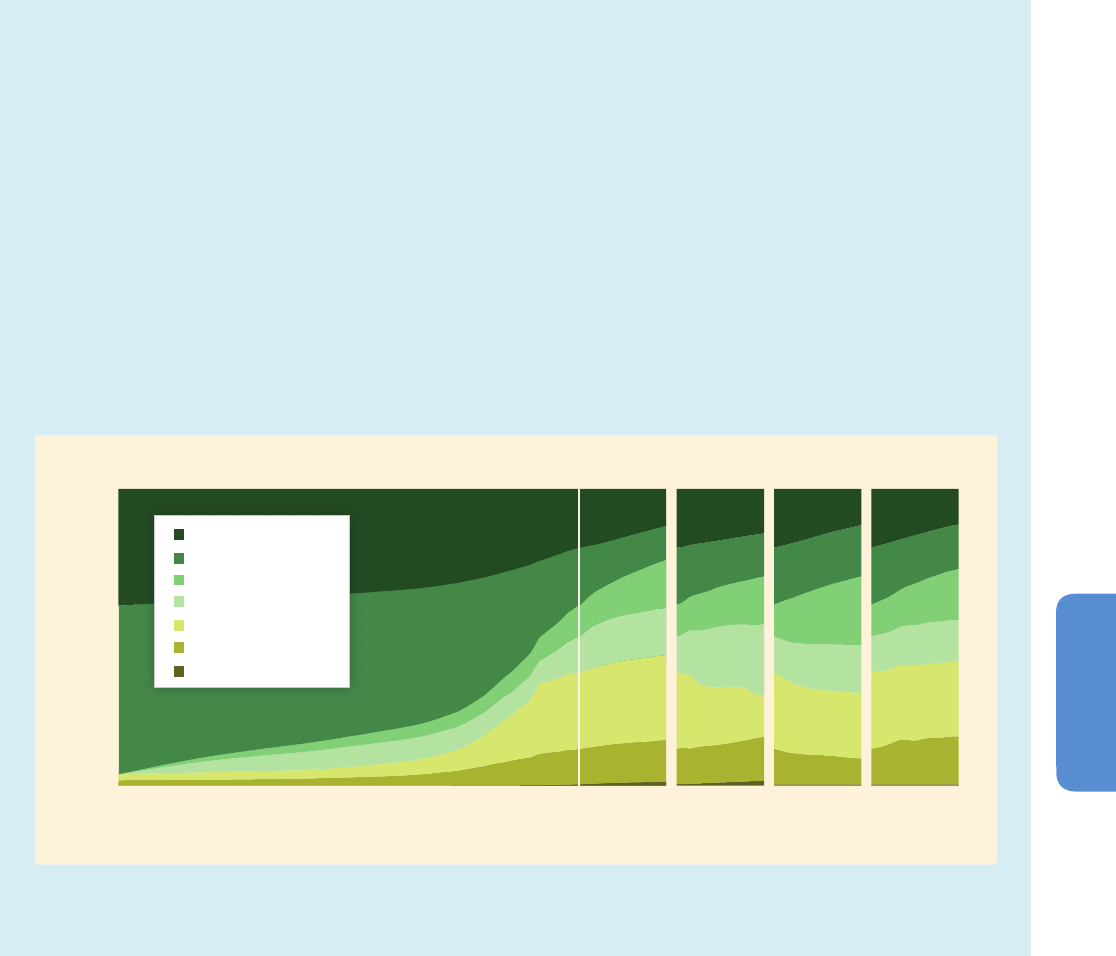
Figure 4-2 | Projections of climate change-driven biome shifts in the context of direct human land use. (a) Fraction of land covered by primary vegetation in 2005 (Hurtt et al.,
2011); (b) Fraction of land covered by primary vegetation in 2100 under the RCP2.6 land use scenario, with no effect of climate change (Hurtt et al., 2011); (c) Fraction of land
covered by primary vegetation in 2100 under the RCP6.0 land use scenario, with no effect of climate change (Hurtt et al., 2011). (d) Fraction of simulations showing climate
change-driven biome shift for any level of global warming between 1990 and 2100, with no direct anthropogenic land use change, using the MC1 vegetation model under 9
CMIP3 climate projections (3 GCMs, each forced by the SRES A2, A1B, and B1 scenarios; Gonzalez et al., 2010); Comparison of colored areas in (d) with those in (a) shows
where climate-driven biome shifts would occur in current areas of primary vegetation. Comparison of (b) and (c) with (a) illustrates two scenarios of how primary vegetation
could change due to direct human land use, irrespective of the effects of climate change. (b) shows the land use scenario associated with RCP2.6, in which global climate
change is projected to be smaller than that driving the biome shifts in (d) as a result of mitigation measures, some of which involved land use. (c) shows the land use scenario
associated with RCP6.0, in which global climate change is projected to be larger than RCP2.6 so biome shifts similar to those in (d) may occur alongside the projected land use
changes in (c). For example, climate change-driven biome shift is projected in many Arctic land areas (d) which are unaffected by direct human land use at the present day (a)
and in the RCP2.6 and 6.0 land use scenarios (b, c), indicating that climate change is the dominant influence on Arctic land ecosystems in these scenarios. In contrast, in Borneo,
none of the GCMs analysed by Gonzalez et al. (2010) project climate change-driven biome shift (d), and instead a reduction in primary vegetation cover occurs in the mitigation
scenario RCP2.6 as a consequence of direct human land use (b). A smaller reduction occurs in RCP6.0. Land use is therefore projected to be the dominant driver of change in
Borneo in these scenarios. In the boreal forest regions of North America, Europe, and north-west Asia, climate change-driven biome shift (d) is projected in regions already
subject to some influence of present-day human land use (a), and increased land use leading to further reductions in primary vegetation occur in both RCP2.6 (b) and RCP6.0
(c). Hence in these boreal forest regions, both climate change and land use are projected to be drivers of ecosystem change in these scenarios. Further details of the RCP land
use/cover scenarios are given in Box 4-1, Figure 4-3, and Table 4-2.

282
Chapter 4 Terrestrial and Inland Water Systems
4
p
reindustrial in some continental-scale regions; see WGI AR5 Section
5.5.1) was the same order of magnitude as the warming the Earth has
experienced over the 20th century. Ecological effects were small
compared to periods with larger climate excursions, but even this small
warming was characterized by frequent fires in drier parts of the Amazon
(Mayle and Power, 2008), development of lush vegetation and lakes in
a wetter Sahara (Watrin et al., 2009), temperate deciduous forests in
Europe expanding further north and up to higher elevations (Prentice
et al., 1996), and large-scale migration of Boreal Forest into a warmer
tundra (Jackson and Overpeck, 2000). Past climate change, even more
modest than mid-range projected future change, also clearly impacted
inland water systems (e.g., Smol and Douglas, 2007a; Battarbee et al.,
2009; Beilman et al., 2009). However, there are no exact analogs for
future climate change: none of the well-studied past periods of large
climate change involved simultaneously the rates, magnitude, and
spatial scale of climate and atmospheric carbon dioxide (CO
2
) change
projected for the 21st century and beyond (Jansen et al., 2007; Schulte
et al., 2010; Wing and Currano, 2013; see WGI AR5 Chapter 5). Direct
analogy with the paleoecological record is also unwarranted because
future climate change will interact with other global changes such as
land use change, invasive species, pollution, and overexploitation of
natural resources (Pereira et al., 2010). There is high confidence that
these interactions will be important: the paleoecological record provides
medium confidence (medium evidence, high agreement) that exploitation
by humans helped drive many large mammal species to extinction during
periods of climate change in the past (Lorenzen et al., 2011).
It has been demonstrated that state-of-the-art vegetation models are able
to simulate much of the biome-level equilibrium response of terrestrial
vegetation to large paleoclimate change (Prentice et al., 1996, 2011;
Salzmann et al., 2008). The same types of models predict large changes in
species ranges, ecosystem function, and carbon storage when forced by
21st century climate change, although the future situation is complicated
by land use and other factors absent in the paleoenvironmental case
(Sitch et al., 2008; Cheaib et al., 2012; see WGI AR5 Section 6.4). Thus,
the paleoecological record and models that have been tested against it
provide a coherent message that biomes will alter their functioning and
composition in response to changing and often novel future climates:
they will move as species mixtures change (Section 4.3.2.5 has more
specific information on projected migration rates), novel plant communities
will emerge, and significant carbon stock changes will take place
(Williams and Jackson, 2007; MacDonald, 2010; Prentice et al., 2011;
W
illis and MacDonald, 2011). The paleoecological record and models
provide high confidence that it will be difficult or impossible to maintain
many ecological systems in their current states if global warming exceeds
2°C to 3°C, raising questions about the long-term viability of some
current protected areas and conservation schemes, particularly where
the objective is to maintain present-day species mixtures (Jackson and
Hobbs, 2009; Hickler et al., 2012).
Much of the complex, time-dependent change at regional scales has
not yet been simulated by models. The paleoecological record indicates
that vegetation in many parts of the world has the potential to respond
within years to a few decades to climate change (e.g., Mueller, A.D. et al.,
2009; Watrin et al., 2009; Williams et al., 2009; Harrison and Goni, 2010).
This record provides a critical opportunity for model evaluation that
should be more thoroughly exploited to gain confidence in time-
dependent simulations of future change, particularly given the complex
role that interacting climate change and vegetation disturbance has
played in the past (e.g., Jackson et al., 2009; Marlon et al., 2009;
Williams et al., 2009; Daniau et al., 2010; Dawson et al., 2011). The
paleoecological record also highlights the importance of including the
direct effects of changing atmospheric CO
2
levels in efforts to simulate
future ecosystem functioning and plant species competition (Prentice
et al., 2011; Woillez et al., 2011; Bond and Midgley, 2012; Claussen et
al., 2013).
The paleoeclimatic record also reveals that past radiative climate forcing
change was slower than that anticipated for the 21st century (see WGI
AR5 Chapters 5, 8, and 12), but even these slower changes often drove
surprisingly abrupt, nonlinear, regional-scale change in terrestrial and
inland water systems (e.g., Harrison and Goni, 2010; Williams et al.,
2011), as did even slower climate change during the most recent
Holocene interglacial (e.g., Booth et al., 2005; Kropelin et al., 2008;
Williams, J.W. et al., 2010; Williams et al., 2011). In all cases, specific
periods of abrupt ecological response were regionally distinct in nature
and were less synchronous for small, slow changes in forcing (e.g.,
during the Holocene) than for the global-scale rapid changes listed at
the start of this section. State-of-the-art climate and Earth System
Models (ESMs) are unable to simulate the full range of abrupt change
observed in many of these periods (e.g., Valdes, 2011). Thus there is
high confidence that these models may not capture some aspects of
future abrupt climate change and associated ecosystem impacts (Leadley
et al., 2010).
Frequently Asked Questions
FAQ 4.1 | How do land use and land cover changes cause changes in climate?
Land use change affects the local as well as the global climate. Different forms of land cover and land use can cause
warming or cooling and changes in rainfall, depending on where they occur in the world, what the preceding land
cover was, and how the land is now managed. Vegetation cover, species composition, and land management practices
(such as harvesting, burning, fertilizing, grazing, or cultivation) influence the emission or absorption of greenhouse
gases. The brightness of the land cover affects the fraction of solar radiation that is reflected back into the sky, instead
of being absorbed, thus warming the air immediately above the surface. Vegetation and land use patterns also influence
water use and evapotranspiration, which alter local climate conditions. Effective land use strategies can also help to
mitigate climate change.

283
Terrestrial and Inland Water Systems Chapter 4
4
4.2.4. Multiple Stressors Interacting with Climate Change
The climatic and non-climatic drivers of ecosystem change need to be
distinguished if the joint and separate attribution of changes to their
causes is to be performed (see Chapter 18). In this section we elaborate
on factors affecting ecosystems, operating simultaneously with climate
change. These factors share underlining drivers with one another and
with climate change to varying degrees; together they form a syndrome
known as “global change.” The individual effects of climate change,
habitat loss and fragmentation, chemical pollution, overharvesting, and
invasive alien species are increasingly well documented (Millennium
Ecosystem Assessment, 2005c; Settele et al., 2010a) but much less is
known about their combined consequences. Ecosystem changes may
occur in cascades, where a change in one factor precipitates increased
vulnerability with respect to other factors (Wookey et al., 2009) or
propagates through the ecosystem as a result of species interactions
(Gilman et al., 2010). Multiple stressors can act in a non-additive way
(Shaw et al., 2002; Settele et al., 2010b; Larsen et al., 2011), potentially
invalidating findings and interventions based on single-factor analysis.
For instance, Larsen et al. (2011) demonstrated that non-additive
interactions among the climate factors in a multifactor experiment were
frequent and most often antagonistic, leading to smaller effects than
predicted from the sum of single factor effects. Leuzinger et al. (2011)
and Dieleman et al. (2012) have synthesized multifactor experiments
and demonstrated that, in general, the effect size is reduced when more
factors are involved, but Leuzinger et al. (2011) suggest that multifactor
models tend to show the opposite tendency.
4.2.4.1. Land Use and Cover Change
Land use and cover change (LUCC) is both a cause (WGI AR5 Section
6.1.2) and a consequence of climate change. It is the major driver of
current ecosystem and biodiversity change (Millennium Ecosystem
Assessment, 2005b) and a key cause of changes in freshwater systems
(Section 4.3.3.3). In tropical and subtropical areas of Asia, Africa,
Oceania, and South America, the dominant contemporary changes are
conversion of forests and woodlands to annual and perennial agriculture,
grazing pastures, industrial logging, and commercial plantations,
followed by conversion of savannas, grasslands, and pastures to annual
agriculture (Hosonuma et al., 2012; Macedo et al., 2012). In Europe
there is net conversion of agricultural lands to forest (Rounsevell and
Reay, 2009; Miyake et al., 2012). Conversion of peatlands to agriculture
has been an important source of carbon to the atmosphere in Southeast
Asia (Limpens et al., 2008; Hooijer et al., 2010; see Section 4.3.3.3).
Contemporary drivers of LUCC include rising demand for food, fiber, and
bioenergy and changes in lifestyle and technologies (Hosonuma et al.,
2012; Macedo et al., 2012). By mid-century climate change is projected
to become a major driver of land cover change (Leadley et al., 2010).
Non-climate environmental changes such as nitrogen deposition, air
pollution, and altered disturbance regimes are also implicated in LUCC.
Some of the underlying drivers of LUCC are also direct or indirect drivers
of climate change (Cui and Graf, 2009; McAlpine et al., 2009; Mishra et
al., 2010; Schwaiger and Bird, 2010; van der Molen et al., 2011; Groisman
et al., 2012); this cause-and-effect entanglement of climate change and
LUCC can confound the detection of climate change and make attribution
t
o one or the other difficult. Local-to-regional climate change was at
least partly attributed to LUCC in 11 of 26 studies reviewed for this
chapter, generally with limited evidence and low confidence. (Direct
climate effects attributed to LUCC: Cui and Graf, 2009; Li et al., 2009;
McAlpine et al., 2009; Zhang et al., 2009; Fall et al., 2010; Jin et al.,
2010; Mishra et al., 2010; Schwaiger and Bird, 2010; Wu et al., 2010;
Carmo et al., 2012; Groisman et al., 2012. No climate effects studied:
Suarez et al., 1999; Saurral et al., 2008; Tseng and Chen, 2008; Wang et
al., 2008; Cochrane and Barber, 2009; Jia, B. et al., 2009; Rounsevell and
Reay, 2009; Graiprab et al., 2010; Martin et al., 2010; Wiley et al., 2010;
Clavero et al., 2011; Dai et al., 2011; Gao and Liu, 2011; Viglizzo et al.,
2011; Yoshikawa and Sanga-Ngoie, 2011).
LUCC (and land use itself) contributes to changes in the climate through
altering the GHG concentrations in the atmosphere, surface and cloud
albedos, surface energy balance, wind profiles, and evapotranspiration,
among other mechanisms. The phrase “biophysical effects” is shorthand
for the effect vegetation has on the climate other than through its role
as a source or sink of GHGs. These effects are now well documented,
significant, and are increasingly included in models of global and regional
climate change. The GHG and biophysical effects of vegetation can be
opposite in sign (de Noblet-Ducoudre et al., 2012) and operate at
different scales. For instance, conversion of forest to non-forest generally
releases CO
2
from biomass and soils to the atmosphere (causing warming
globally), but may result in an increase in seasonally averaged albedo
(local and global cooling, Davin et al., 2007) and a decrease in
transpiration (local, but not global warming). Findell et al. (2007)
concluded on the basis of model studies that the non-GHG climate
impacts of LUCC were generally minor, but nevertheless significant in
some regions. Brovkin et al. (2013), projecting the overall effect of LUCC
on climate change for the 21st century, found LUCC to be a small driver
globally, but locally important. Most global climate models suggest
local average cooling effects following forest conversion to croplands
and pastures (Pitman et al., 2009; Longobardi et al., 2012). Satellite
observations suggest that the effect of conversion of the Brazilian savannas
(cerrado) to pasture was to induce a local warming that was partly
reversed when the pasture was subsequently converted to sugarcane
(Loarie et al., 2011). Several modeling studies suggest that the global
surface air temperature response to deforestation depends on the latitude
at which deforestation occurs. High-latitude deforestation results in
global cooling, low-latitude deforestation causes global warming, and
the mid-latitude response is mixed (Bathiany et al., 2010; Davin and de
Noblet-Ducoudre, 2010; van der Molen et al., 2011; Longobardi et al.,
2012), with some exceptions documented for boreal forests (Spracklen
et al., 2008). Boreal and tropical forests influence the climate for different
reasons: boreal forests have low albedo (i.e., reflect less solar radiation,
especially in relation to a snowy background; Levis, 2010; Mishra et al.,
2010; Longobardi et al., 2012) and tropical forests pump more water
and aerosols into the atmosphere than non-forest systems in similar
climates (Davin and de Noblet-Ducoudre, 2010; Delire et al., 2011; Pielke
et al., 2011). The implications of these findings for afforestation as a
climate mitigation action are discussed in Section 4.3.4.5. Forests may
also influence regional precipitation through biophysical effects (Butt
et al., 2011; Pielke et al., 2011; see Section 4.3.3).
In summary, changes in land cover have biophysical effects on the
climate, sometimes opposite in direction to GHG-mediated effects,
284
Chapter 4 Terrestrial and Inland Water Systems
4
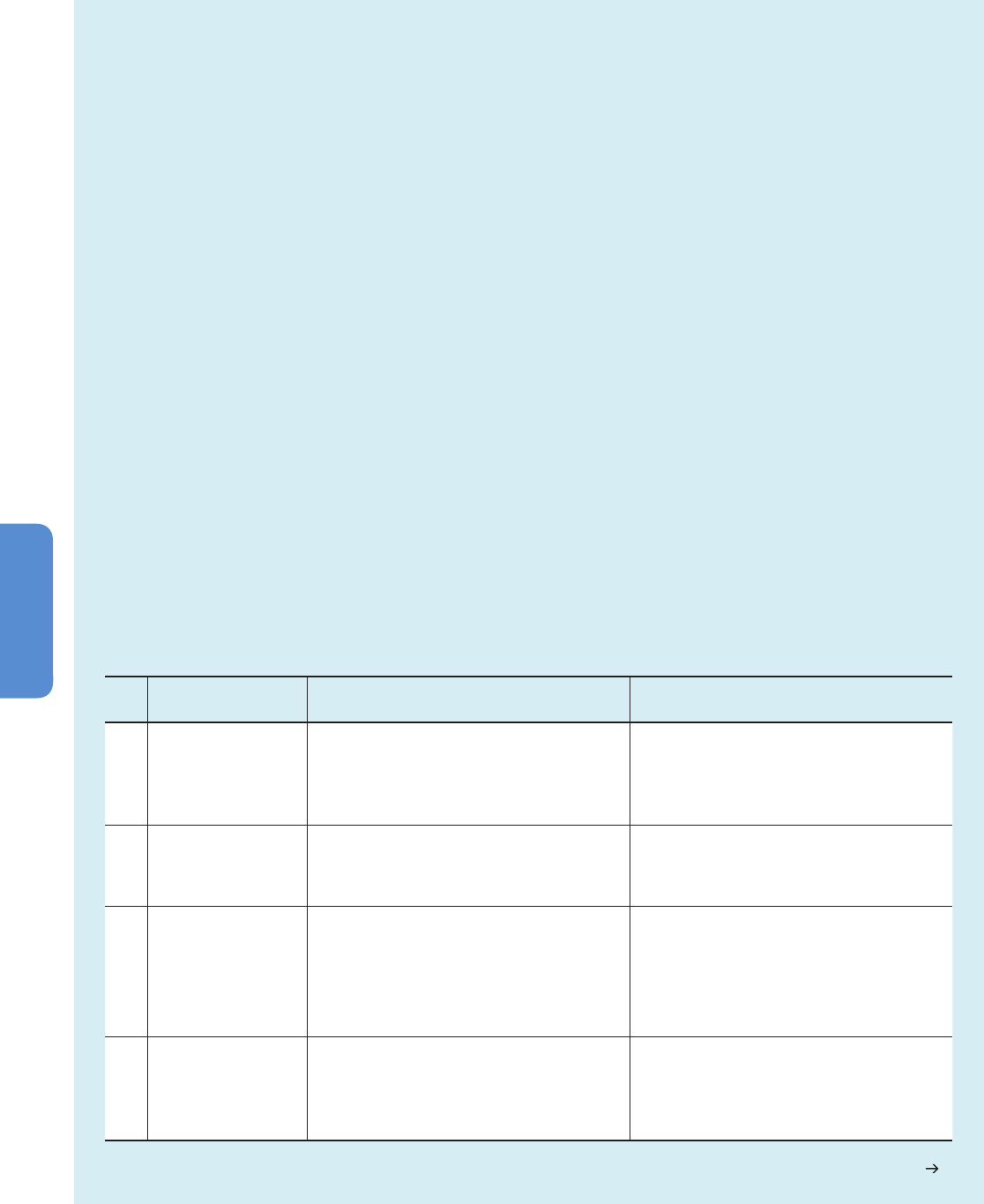
Box 4-1 | Future Land Use Changes
Assessment of climate change effects on terrestrial and inland freshwater ecosystems requires the simultaneous consideration of
land use and cover change (LUCC). The world is undergoing important shifts in land use, driven by accelerating demand for food,
feed, fiber, and fuel. The main underlying driver is the rate at which per capita consumption is growing, particularly in emerging
economies (Tilman et al., 2011). Policy shifts in developed countries favoring biofuel production have also contributed (Searchinger et
al., 2008; Lapola et al., 2010; Miyake et al., 2012). Agricultural commodity prices have risen and may stay high through 2020 (OECD
and FAO, 2010), owing to (1) demand growth outpacing supply growth, exacerbated by climate-related crop failure (Lobell et al.,
2011); (2) decline in the rate of improvement in agricultural productivity (Ray et al., 2012); (3) shortage of arable land not already
under cultivation, especially in the temperate zone; (4) growing pressure on as-yet uncultivated ecosystems on soils that are potentially
suitable for cultivation and that are concentrated in tropical latitudes, especially South America and Africa (Lambin and Meyfroidt,
2011); and (5) declining area under cultivation in temperate zones, mainly in developed countries. The shortage of arable land in
temperate systems could put pressure on marginal or sensitive landscapes, mainly in Latin America’s cerrados and grasslands (Brazil,
Argentina) and in African savannas (Sudan, Democratic Republic of Congo, Mozambique, Tanzania, Madagascar) (Lambin and
Meyfroidt, 2011).
Deforestation in developing countries correlates with the export of agricultural commodities (DeFries et al., 2010). Future LUCC
remains uncertain, as it depends on economic trends and policies themselves dependent on complex political and social processes,
including climate policy. By 2012, the deforestation rate in the Brazilian Amazon had declined by 77% below its 1996–2005 average
(Nepstad et al., 2009; INPE, 2013) as a result of policy and market signals (Soares-Filho et al., 2010). This single trend represents a
1.5% reduction in global anthropogenic carbon emissions (Nepstad et al., 2013).
RCP Model and references Key assumptions /drivers Land use /cover outcomes
8.5 MESSAGE; Riahi et al. (2007) • No climate change mitigation actions; radiative forcing still
rising at 2100.
• Strong increase in agricultural resource use driven by the
increasing population (rises to 12 billion people by 2100).
• Yield improvements and intensifi cation assumed to account for
most of production increases.
• Increase in cultivated land by about 305 million ha from 2000
to 2100.
• Forest cover declines by 450 million ha from 2000 to 2100.
• Arable land use in developed countries slightly decreased — all
of the net increases occur in developing countries.
6.0 AIM; Fujino et al. (2006),
Hijioka et al. (2008)
• Mitigation actions taken late in the century to stabilize radiative
forcing at 6 W m
−2
after 2100.
• Population growth and economic growth.
• Increasing food demand drives cropland expansion .
• Urban land use increases.
• Cropland area expands.
• Grassland area declines.
• Total forested area extent remains constant.
4.5 GCAM; Smith and Wigley
(2006), Wise et al. (2009)
• Mitigation stabilizes radiative forcing at 4.5 W m
−2
before 2100.
• Assumes that global greenhouse gas emissions prices are
invoked to limit emissions and therefore radiative forcing.
Emissions pricing assumes all carbon emissions are charged an
equal penalty price, so reductions in land use change carbon
emissions available as mitigation.
• Food demand is met through crop yield improvements, dietary
shifts, production effi ciency, and international trade.
• Preservation of large stocks of terrestrial carbon in forests.
• Overall expansion in forested area.
• Agricultural land declines slightly due to afforestation.
2.6 IMAGE; van Vuuren et al.
(2006), van Vuuren et al. (2007)
• Overall trends in land use and land cover are determined mainly
by demand, trade, and production of agricultural products and
bioenergy.
• Expansion of croplands largely due to bioenergy production.
• Production of animal products is met through shift from
extensive to more intensive animal husbandry.
• Much agriculture relocates from high-income to low-income
regions.
• Increase in bioenergy production, new area for bioenergy crops
near current agricultural areas.
• Pasture largely constant.
Table 4-2 | Summary of drivers and outcomes of Land Use and Land Cover Change (LUCC) scenarios associated with Representative Concentration Pathways (RCPs;
Hurtt et al., 2011). RCPs are identifi ed with the radiative forcing by 2100 (8.5, 6.0, 4.5, and 2.6 W m
–2
) and by the name of the model used to generate the associated
land use /cover scenarios (MESSAGE (Model for Energy Supply Strategy Alternatives and their General Environmental Impact), AIM (Asia-Pacifi c Integrated Model),
GCAM (Global Change Assessment Model), and IMAGE (Integrated Model to Assess the Global Environment); see Hurtt et al. (2011) for further details).
Continued next page
285
Terrestrial and Inland Water Systems Chapter 4
4
which can materially alter the net outcome of the land cover change
on the global climate (high confidence).
4.2.4.2. Nitrogen Deposition
The global nitrogen cycle has been strongly perturbed by human activity
over the past century (Gruber and Galloway, 2008; Canfield et al., 2010).
Activities such as fertilizer production and fossil fuel burning currently
transform 210 TgN yr
–1
of nitrogen gas in the atmosphere into reactive
forms of nitrogen (N
r
) that can be readily used by plants and
microorganisms in land and in the ocean, slightly more than the non-
anthropogenic transformation of 203 TgN yr
–1
(Fowler et al., 2013). Most
of the transformations of anthropogenic N
r
are on land (Fowler et al.,
2013). The human-caused flow from land to oceans in rivers is 40 to
70 TgN yr
–1
, additional to the estimated natural flux of 30 TgN yr
–1
(Galloway et al., 2008; Fowler et al., 2013). Many of the sources of
additional nitrogen share root causes with changes in the carbon cycle,
such as increased use of fossil fuels and expansion and intensification
of global agriculture. Nitrogen deposition, CO
2
concentrations, and
temperatures are therefore increasing together at global scales (Steffen
et al., 2011). Regional trends in nitrogen fluxes differ substantially:
nitrogen fertilizer use and nitrogen deposition are stable or declining
in some regions, such as Western Europe; but nitrogen deposition and
its impacts on biodiversity and ecosystem functioning are projected to
increase substantially over the next several decades in other regions,
especially in the tropics (Galloway et al., 2008) owing to increased
needs for food and energy for growing populations in emerging
economies (e.g., Zhu et al., 2005).
Experiments and observations, most of which are in temperate and boreal
Europe and North America, show a consistent pattern of increase in the
Box 4-1 (continued)
Each of the four main Representative Concentration Pathways (RCPs) used for future climate projections has a spatially explicit future
land use scenario consistent with both the emissions scenario and the underlying associated socioeconomic scenario simulated by
integrated assessment models, as well as conditions in 2005 (Hurtt et al., 2011; see also Table 4-2, Figure 4-2, Figure 4-3). In scenarios
where cropland and pasture are projected to decrease, they are replaced with secondary vegetation. Tropical and boreal forest regions
are both projected to undergo declining primary forest cover in most RCPs, but in RCP6.0 total forest area remains approximately
constant and in RCP4.5 total forest area expands because of increased secondary forest. The extent to which primary vegetation is
replaced by secondary vegetation, crops, or pasture varies between the RCPs (Figure 4-3), with no simple linear relationship between
the extent of vegetation change and the level of total radiative forcing. Larger reductions in primary vegetation cover are projected in
RCP8.5, owing to a general absence of proactive measures to control land cover change in that scenario. Large reductions are also
projected in RCP2.6 owing to widespread conversion of land to biofuel crops (Figure 4-2). Smaller reductions are foreseen in RCP6.0
and RCP4.5, with the latter involving conservation of primary forest and afforestation as mitigation measures.
RCP8.5
2000 2100 2000 2100 2000 2100
RCP6.0 RCP4.5 RCP2.6Historical
Year
1500 1600 1700 1800 1900 2000 2100
Fraction of global land area
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
Urban
Cropland
Pasture
Secondary non-forest
Secondary forest
Primary non-forest
Primary forest
Figure 4-3 | Proportion of global land cover occupied by primary and secondary vegetation (forest and non-forest), cropland, pasture, and urban land, from satellite
data and historical reconstructions up to 2005 (Klein Goldewijk et al., 2010, 2011), and from scenarios associated with the RCPs from 2005 to 2100 (Hurtt et al., 2011).

286
Chapter 4 Terrestrial and Inland Water Systems
4
d
ominance of a few nitrogen-loving plant species and loss of overall
plant species richness at nitrogen deposition loads exceeding between 5
and 20 kgN ha
–
1
yr
–
1
(Power et al., 2006; Clark and Tilman, 2008; Bobbink
et al., 2010; but see Stevens, C.J. et al., 2010). Nitrogen deposition is
currently above these limits in much of Europe, eastern North America,
and southern Asia (Galloway et al., 2008), including in many protected
areas (Bleeker et al., 2011).
The impacts of nitrogen deposition are often first manifested in freshwater
ecosystems because they collect and concentrate the excess nitrogen
(and phosphorus) from the land, as well as from sewage and industrial
effluents. Primary production in freshwater ecosystems can be either
nitrogen and phosphorus limited or both (Elser et al., 2007), but the
biodiversity and capacity of freshwater ecosystems to deliver high-
quality water, recreational amenity, and fisheries services is severely
reduced by the addition of nutrients beyond their capacity to process
them. Excessive loading of nitrogen and phosphorus is widespread in
the lakes of the Northern Hemisphere (NH; Bergström and Jansson,
2006), although reduced nitrogen loading including deposition was
observed between 1988 and 2003 in Sweden (Weyhenmeyer et al.,
2007). The observed symptoms include a shift from nitrogen limitation
of phytoplankton in lakes to phosphorus limitation (Elser et al., 2009).
Since the AR4, an increasing number of studies have models, observations,
and experiments to understand and predict the interactive effects of
nitrogen deposition, climate change, and CO
2
on ecosystem function.
Interactions between nitrogen and other global change factors are
widespread, strong, and complex (Rustad, 2008; Thompson et al., 2008;
Langley and Megonigal, 2010; Gaudnik et al., 2011; Eisenhauer et al.,
2012; Hoover et al., 2012; but see Zavaleta et al., 2003, for evidence of
additive effects). In a study of plant-pollinator relationships, the
combination of nitrogen deposition, CO
2
enrichment, and warming
resulted in larger negative impacts on pollinator populations than could
be predicted from the individual effects (Hoover et al., 2012). In a
perennial grassland species, nitrogen limitation constrained the response
to rising CO
2
(Reich et al., 2006). Broadly, the overall body of research
shows that ecosystem function is mediated by complex interactions
between these factors, such that many ecosystem responses remain
difficult to understand and predict (Churkina et al., 2010; Norby and
Zak, 2011).
In forests in many parts of the world, experiments, observations, and
models suggest that the observed increase in productivity and carbon
storage is due to combinations of nitrogen deposition, climate change,
fertilization effects of rising CO
2
, and forest management (Huang et al.,
2007; Magnani et al., 2007; Pan et al., 2009; Churkina et al., 2010;
Bellassen et al., 2011; Bontemps et al., 2011; de Vries and Posch, 2011;
Eastaugh et al., 2011; Norby and Zak, 2011; Shanin et al., 2011; Lu et
al., 2012). N deposition and rising CO
2
appear to have generally
dominated in much of the NH. However, the direct effects of rising
temperature and changes in precipitation may exceed nitrogen and CO
2
as key drivers of ecosystem primary productivity in a few decades time.
In grasslands, however, experiments show that plant productivity is
increased more by nitrogen addition (within the projected range for
this century) than by elevated CO
2
, also within its projected range, and
that nitrogen effects increase with increasing precipitation (Lee et al.,
2010).
I
n contrast to forests and temperate grasslands, nitrogen deposition and
warming can have negative effects on productivity in other terrestrial
ecosystems, such as moss-dominated ecosystems (Limpens et al., 2011).
The interactions between nitrogen deposition and climate change remain
difficult to understand and predict (Menge and Field, 2007; Ma et al.,
2011), in part owing to shifts in plant species composition (Langley and
Megonigal, 2010) and the complex dynamics of coupled carbon, nitrogen,
and phosphorus cycles (Menge and Field, 2007; Niboyet et al., 2011).
Analyses using the multi-factor biodiversity change model GLOBIO3
suggest that nitrogen deposition will continue to be a significant
contributing factor to terrestrial biodiversity loss in the first third of the
21st century but will be a less important factor than climate change in
this period, and a much smaller driver than habitat loss due to expansion
of agricultural lands (Alkemade et al., 2009). Models that explicitly take
into account interactive effects of climate change and nitrogen deposition
on plant communities project that nitrogen deposition impacts will
continue to be important, but climate change effects will begin to
dominate other factors by the middle of the 21st century (Belyazid et
al., 2011).
4.2.4.3. Tropospheric Ozone
The concentration of ozone in the troposphere (the part of the atmosphere
adjacent to the Earth’s surface) has risen over the past 150 years from
a global average of 20 to 30 ppb to 30 to 50 ppb, with high spatial and
temporal variability (Horowitz, 2006; Oltmans et al., 2006; Cooper et al.,
2010; WGI AR5 Figure 2.7). This is due to (1) increasing anthropogenic
emissions of gases that react in the atmosphere to form ozone (Denman
et al., 2007) and (2) the increased mixing of stratospheric ozone into
the troposphere as a result of climate change (Hegglin and Shepherd,
2009). The key ozone precursor gases are volatile organic compounds
(VOCs) and oxides of nitrogen (NO
x
). Intercontinental transport of these
precursors contributes to rising global background ozone concentrations,
including in regions where local ozone precursor emissions are decreasing
(Dentener et al., 2010). Global sources of VOC are predominantly
biogenic (BVOC), especially forests (Hoyle et al., 2011).
Negative effects of the current levels of ozone have been widely
documented (Mills et al., 2011). A meta-analysis of more than 300
articles addressing the effect of ozone on tree growth (Wittig et al.,
2009)—focused largely on NH temperate and boreal species—
concluded that current levels of tropospheric ozone suppress growth
by 7% relative to preindustrial levels. Modeling studies that extrapolate
experimentally measured dose-response relationships suggest a 14 to
23% contemporary reduction in Gross Primary Productivity (GPP)
worldwide, with higher values in some regions (Sitch et al., 2007) and
1 to 16% reduction of Net Primary Productivity (NPP) in temperate
forests (Ainsworth et al., 2012).
The mechanisms by which ozone (O
3
) affects plant growth are now better
known (Hayes et al., 2007; Ainsworth et al., 2012). Chronic exposure to
O
3
at levels above about 40 ppb generally reduces stomatal conductance
and impairs the activity of photosynthetic enzymes (The Royal Society,
2008), although in some cases ozone exposure increases stomatal
conductance (Wilkinson and Davies, 2010). For the species studied,

287
Terrestrial and Inland Water Systems Chapter 4
4
c
arbon assimilation rates and leaf area are generally reduced, while
respiration increases and leaf senescence is accelerated—all leading to
a reduction in NPP. Conifers are less sensitive than broad-leafed species.
In a modeling study, lower stomatal conductance due to O
3
exposure
increased river runoff by reducing the loss of soil moisture through
transpiration, but observational studies that measured runoff in relation
to ozone exposure show divergent trends on this issue (McLaughlin et
al., 2007; Wittig et al., 2007; Mills et al., 2009; Huntingford et al., 2011).
A modeling study (Sitch et al., 2007) suggests that the negative effects
of rising O
3
on plant productivity could offset 17 to 31% of the projected
increase in global carbon storage due to increasing CO
2
concentrations
over the 21st century, but the possible interactive effects between CO
2
and O
3
are poorly understood (The Royal Society, 2008). Reduced
stomatal conductance, widely observed under elevated CO
2
, should help
protect plants from ozone damage. Some chamber experiments
(Bernacchi et al., 2006) and model studies (Klingberg et al., 2011)
suggest this to be the case. The one plot-scale study of CO
2
and O
3
interactions in a temperate forest (Karnosky et al., 2005; Hofmockel et
al., 2011) suggests that the effects of O
3
and CO
2
are not independent
and may partly compensate for one another.
There is genotypic variation in plant sensitivity to O
3
(Ainsworth et al.,
2012). Other than changing cultivars or species, few management actions
promoting adaptation to higher levels of O
3
are currently available
(Wilkinson and Davies, 2010; Teixiera et al., 2011). Research into
developing ozone resistant varieties and chemical protectants against
damage may provide management options in the future (Wilkinson and
Davies, 2010; Ainsworth et al., 2012).
4.2.4.4. Rising Carbon Dioxide
Rising atmospheric CO
2
concentrations affect ecosystems directly and
through biological and chemical processes. The consequences for the
global carbon cycle are discussed in WGI AR5 Box 6.3; the discussion
here focusses on impacts on terrestrial and inland water systems. Paleo
records over the Late Quaternary (past Myr) show that changes in the
atmospheric CO
2
content between 180 and 280 ppmv had ecosystem-
scale effects worldwide (Prentice and Harrison, 2009).
In contrast to the oceans, changes in CO
2
concentrations in inland
waters are influenced primarily by biological processes, such as inputs
o
f terrestrial organic matter, particularly dissolved organic carbon (DOC),
and bacterial respiration (van de Waal et al., 2010; Aufdenkampe et al.,
2011). Carbon can, however, become limiting during intense algal
blooms, especially in the surface waters of stratified lakes and reservoirs,
and rising atmospheric CO
2
concentrations may stimulate higher algal
production under these conditions (van de Waal et al., 2010). Higher
CO
2
concentrations can lead to increases in the C:N and C:P ratios of
phytoplankton, though the trophic consequences of this are difficult to
predict because zooplankton may alter their feeding behavior to select
higher quality forms of algae or increase feeding rate (Urabe et al., 2003;
van de Waal et al., 2010).
Over the past 2 decades, and especially since AR4, experimental
investigation of elevated CO
2
effects on plants and ecosystems has used
mainly Free Air CO
2
Enrichment (FACE) techniques (Leakey et al., 2009).
FACE is considered more realistic than earlier approaches using enclosed
chambers, because plant community and atmospheric interactions and
below-ground conditions are more like those of natural systems. Plants
with a C
3
photosynthetic system, which includes most species but
excludes warm-region grasses, show an increase in photosynthesis
under elevated CO
2
, the precise magnitude of which varies between
species. Acclimation (“down-regulation”) occurs under long-term
exposure, leading to cessation of effects in some (Norby and Zak, 2011)
but not all studies (Leakey et al., 2009). The C
4
photosynthetic system
found in most tropical grasses and some important crops is not directly
affected by elevated CO
2
, but C
4
plant productivity generally increases
under elevated CO
2
because of increased water use efficiency (WUE).
Transpiration is decreased under elevated CO
2
in many species, due to
reduced opening of stomatal apertures, leading to greater WUE (Leakey
et al., 2009; Leuzinger and Körner, 2010; De Kauwe et al., 2013).
Increasing WUE is corroborated by studies of stable carbon isotopes
(Barbosa et al., 2010; Koehler et al., 2010; Silva et al., 2010; Maseyk et
al., 2011). The WUE increase does not acclimate to higher CO
2
in the
medium term, that is, over several years (Leakey et al., 2009). Satellite
observations from 1982–2010 show an 11% increase in green foliage
cover in warm, arid environments (where WUE is most important) after
correcting for the effects of precipitation variability (Donohue et al.,
2013); gas exchange theory predicts 5 to 10% greening resulting from
rising CO
2
over this period.
The interactive effects of elevated CO
2
and other global changes (such as
climate change, nitrogen deposition, and biodiversity loss) on ecosystem
function are extremely complex. Generally, nitrogen use efficiency is
Frequently Asked Questions
FAQ 4.2 | What are the non–greenhouse gas effects of rising carbon dioxide on ecosystems?
Carbon dioxide (CO
2
) is an essential building block of the process of photosynthesis. Simply put, plants use sunlight
and water to convert CO
2
into energy. Higher CO
2
concentrations enhance photosynthesis and growth (up to a point),
and reduce the water used by the plant. This means that water remains longer in the soil or recharges rivers and
aquifers. These effects are mostly beneficial; however, high CO
2
also has negative effects, in addition to causing global
warming. High CO
2
levels cause the nitrogen content of forest vegetation to decline and can increase their chemical
defenses, reducing their quality as a source of food for plant-eating animals. Furthermore, rising CO
2
causes ocean
waters to become acidic (see FAQ 6.3), and can stimulate more intense algal blooms in lakes and reservoirs.

288
Chapter 4 Terrestrial and Inland Water Systems
4
i
ncreased under higher CO
2
(
Leakey et al., 2009) although, in some tree
FACE experiments, productivity increases as a result of enhanced CO
2
if
sustained by increased nitrogen uptake rather than increased nitrogen
use efficiency (Finzi et al., 2007). In one 10-year temperate grassland
experiment in Minnesota, elevated CO
2
halved the loss of species richness
expected from nitrogen addition (Reich, 2009), whereas no such benefit
was reported for an alpine grassland in France (Bloor et al., 2010) or a
Danish heathland ecosystem (Kongstad et al., 2012).
Elevated CO
2
can affect plant response to other stresses, such as high
temperature (Lloyd and Farquhar, 2008) and drought. Ozone exposure
decreases with lower stomatal conductance (Sitch et al., 2007). In
savannas, faster growth rates under higher CO
2
can allow woody plants
to grow tall enough between successive fires to escape the flames
(Bond and Midgley, 2001; Scheiter and Higgins, 2009). Differential
species responses to elevated CO
2
appear to be altering competition
(Dawes et al., 2011), for example, increasing the likelihood of faster-
growing species such as lianas out-competing slower-growing species
such as trees (Mohan et al., 2006; Potvin et al., 2007; Lewis et al., 2009a).
Experimental studies have shown that elevated CO
2
leads to increased
leaf C:N ratios in woody plants, forbs, and C
3
grasses (but not C
4
grasses),
which may decrease their quality as food and increase herbivorous
insect feeding rates and changes to their density and community structure
(Sardans et al., 2012). Plants may also become more toxic to herbivores
under elevated CO
2
levels, through increased concentrations of carbon-
and nitrogen-based defenses (Lindroth, 2010; Cavagnaro et al., 2011).
Our understanding of ecosystem responses to elevated CO
2
is incomplete
in some respects. The majority of FACE experiments apply upper
CO
2
concentrations of approximately 550 ppmv, which is below the
concentrations projected by 2100 under higher emissions scenarios. The
physiology of photosynthesis suggests that direct CO
2
effects saturate
at levels of approximately 700 ppmv (Long et al., 2004). Most elevated
CO
2
experiments impose a sudden increase of CO
2
concentration as
opposed to the gradual rise experienced in reality. Most large-scale
FACE experiments have been conducted in temperate locations (e.g.,
Hickler et al., 2008); there are currently no large-scale tropical or boreal
FACE experiments. The magnitude of CO
2
effects decreases as the spatial
scale of study increases (Leuzinger et al., 2011).The scale of controlled
experiments is limited to approximately 100 m
2
. Extrapolation to larger
scales ignores large-scale atmospheric feedbacks (Körner et al., 2007)
and catchment-scale hydrological effects (see Box CC-VW). Overall,
there is medium confidence (much evidence, medium agreement) that
increases in CO
2
up to about 600 ppm will continue to enhance
photosynthesis and plant water use efficiency, but at a diminishing rate.
CO
2
effects are a first-order influence on model projections of ecosystem
and hydrological responses to anthropogenic climate change (Sitch et
al., 2008; Lapola et al., 2009; Friend et al., 2013).The direct effect of CO
2
on plant physiology, independent of its role as a GHG, means that
assessing climate change impacts on ecosystems and hydrology solely
in terms of global mean temperature rise (or equivalently, expressing
GHG effects solely in terms of radiative forcing) is an oversimplification
(Huntingford et al., 2011; Betts et al., 2012). A 2°C rise in global mean
temperature, for example, may have a different net impact on ecosystems
depending on the change in CO
2
concentration accompanying the rise
(
e.g., Good et al., 2011a). A high climate sensitivity and/or a higher
proportion of non-CO
2
GHGs would imply a relatively low CO
2
rise at
2°C global warming, so the offsetting effects of CO
2
fertilization and
increased water use efficiency would be smaller than for low climate
sensitivity and/or a lower proportion of non-CO
2
GHGs.
4
.2.4.5. Diffuse and Direct Radiation
The quantity and size distribution of aerosols in the atmosphere alters
both the amount of solar radiation reaching the Earth’s surface and the
proportions of direct versus diffuse radiation. In some regions, direct
radiation has been reduced by up to 30 W m
–2
over the industrial era,
with an accompanying increase in diffuse radiation of up to 20 W m
–2
(Kvalevåg and Myhre, 2007). The global mean direct and diffuse radiation
changes due to aerosols are −3.3 and +0.9 W m
−2
, respectively
(Kvalevåg and Myhre, 2007). For a constant total radiation, an increased
fraction received as diffuse radiation theoretically increases net
photosynthesis because a smaller fraction of the vegetation canopy is
light-saturated, making photosynthesis more light efficient at the
canopy scale (Knohl and Baldocchi, 2008; Kanniah et al., 2012). In a
global model that included this effect, an increase in diffuse fraction of
solar radiation due to volcanic and anthropogenic aerosols and cloud
cover was simulated to lead to approximately a 25% increase in the
strength of the global land carbon sink between 1960 and 1999; however,
under a scenario of climate change and decreased anthropogenic
aerosol concentration, this enhancement declined to near zero by the
end of the 21st century (Mercado et al., 2009), All RCPs project
decreased aerosol concentrations due to air quality protection measures,
as already seen in some countries. The influence of the form of radiation
on plant growth and the land carbon budget is a potentially important
unintended consequence of solar radiation management schemes that
involve the injection of aerosols into the stratosphere to reduce radiant
forcing (see WGI AR5 Section 7.7), but this topic is at present insufficiently
researched for adequate assessment.
4.2.4.6. Invasive and Alien Species
Since the IPCC AR4, the number of observations of the spread and
establishment of alien species attributed to climate change has increased
for several taxa (e.g., Walther et al., 2009) and for particular areas,
including mountain tops and polar regions (McDougall et al., 2011;
Chown et al., 2012). Species invasions have increased over the last
several decades (very high confidence), and the aggressive expansion
of plant and animal species beyond their historical range is having
increasingly negative impacts on ecosystem services and biodiversity
(high confidence; Brook, 2008; Burton et al., 2010; McGeoch et al., 2010;
Simberloff et al., 2013). Climate change will exacerbate some invasion
impacts and ameliorate others (Peterson et al., 2008; Bradley et al., 2009;
Britton et al., 2010; Bellard et al., 2013). Although there is increasing
evidence that some species invasions have been assisted by climate
change, there is low confidence that species invasions have in general
been assisted by recent climatic trends because of the overwhelming
importance of human-facilitated dispersal in mediating invasions. The
spread of alien species has several causes, including habitats made
favorable by climate change (Walther et al., 2009), deliberate species

289
Terrestrial and Inland Water Systems Chapter 4
4
transfer, and accidental transfer due to increased global movement of
goods.
In most cases climate change increases the likelihood of the establishment,
growth, spread, and survival of invasive species populations (Dukes et
al., 2009; Walther et al., 2009; Bradley et al., 2010; Huang et al., 2011;
Chown et al., 2012). Some degree of climate/habitat match has been
found to be a prerequisite of establishment success across seven major
plant and animal groups (Hayes and Barry, 2008). A range of alien
species responses and local consequences are expected (e.g., Rahel and
Olden, 2008; Frelich et al., 2012; Haider et al., 2012; West et al., 2012).
Invasive species, compared to native species, may have traits that favor
their survival, reproduction, and adaptation under changing climates;
invasive plants in particular tend to have faster growth rates and are
particularly favored when resources are not limited (medium to high
confidence; van Kleunen et al., 2010; Willis, C.G. et al., 2010; Buswell et
al., 2011; Davidson et al., 2011; Zerebecki and Sorte, 2011; Haider et al.,
2012; Matzek, 2012). Some invasive plants are more drought tolerant
(Crous et al., 2012; Matzek, 2012; Perry et al., 2012), and on average
they have higher overall metabolic rates, foliar nitrogen concentrations,
and photosynthetic rates than their native counterparts (Leishman et
al., 2007).
Extreme climate events provide opportunities for invasion by generating
disturbances and redistributing available resources (Diez et al., 2012) and
changing connectivity between different ecosystems. Current warming
has already enabled many invasive alien species, including plant,
vertebrate, invertebrate, and single-cell taxa, to extend their distributions
into new areas (high confidence for plants and insects; Walther et al.,
2009; Smith et al., 2012). However, population declines and range
contractions are predicted for some invasive species in parts of their
ranges (Bradley et al., 2009; Sobek-Swant et al., 2012; Taylor et al., 2012;
Bertelsmeier et al., 2013). The expansion of invasive species in some areas
and contraction in others will contribute to community reorganization
and the formation of novel ecosystems and interactions in both terrestrial
and freshwater habitats (high confidence; e.g., Britton et al., 2010;
Kiesecker, 2011; Martinez, 2012; see also Section 4.3.2.5). For example,
invasive grasses may be favored over native ones with increasing
temperatures (Parker-Allie et al., 2009; Chuine et al., 2012; Sandel and
Dangremond, 2012).
In a few cases, benefits to biodiversity and society may result from the
interactive effects of climate change and invasive species, such as
increases in resources available to some threatened species (Caldow et al.,
2007), forest structural recovery (Bolte and Degen, 2010), and available
biomass for timber and fuel (van Wilgen and Richardson, 2012). The
effect of invasions on net changes in carbon stocks are situation specific
and may be either positive or negative (Williams, A.L. et al., 2007). Rising
CO
2
levels will increase the growth rates of most invasive plant species
(Mainka and Howard, 2010; but see Section 4.2.4.4).The effectiveness
of invasive alien species management for sequestering carbon is uncertain
and context specific (Peltzer et al., 2010). Longer term, indirect effects
of invasive alien species will be more important than direct, short-term
effects, for instance, as a result of changes in soil carbon stocks and tree
community composition (low to medium confidence; Peltzer et al., 2010).
Synergistic interactions occur between climate change and invasive
alien species, along with landscape change, habitat disturbance, and
human-facilitated breakdown of dispersal barriers (Brook et al., 2008;
Angeler and Goedkoop, 2010; Bradley et al., 2010; Winder, M. et al.,
2011). Climate change and invasive alien plant species generally
increase the risk and intensity of fire, and the interaction is being
reported more frequently as a direct result of higher temperatures and
increased invasive plant biomass (high confidence; Abatzoglou and
Kolden, 2011). In freshwater systems, alien species establishment and
survival, species interactions, and disease virulence will change as a
result of changes in frequency of high-flow events, increasing water
temperature, water properties, and water demand (medium confidence;
Schnitzler et al., 2007; Rahel and Olden, 2008; Britton et al., 2010).
A range of climate change-related variables (extreme events and changes
in precipitation, temperature, and CO
2
) will continue to exacerbate the
establishment and spread of pests, vectors, and pathogens and negatively
impact production systems (medium confidence; Robinet and Roques,
2010; Clements and Ditommaso, 2011). Warming has contributed to the
spread of many invasive insect species, such as the mountain pine
Frequently Asked Questions
FAQ 4.3 | Will the number of invasive alien species increase as a result of climate change?
Some invasive plants and insects have already been shown to benefit from climate change and will establish and
spread into new regions (where they are “aliens”), once they are introduced. The number of newly arrived species
and the abundance of some already established alien species will increase because climate change will improve
conditions for them. At the same time, increasing movement of people and goods in the modern world, combined
with land use changes worldwide, increases the likelihood that alien species are accidentally transported to new
locations and become established there. There are many actions that can be taken to reduce, but not eliminate, the
risk of alien species invasions, such as the treatment of ballast water in cargo ships and wood products, strict quarantine
applied to crop and horticultural products, and embargos on the trade and deliberate introduction of known invader
species. Some invasive species will suffer from climate change and are expected to decrease in range and population
size in some regions. Generally, increased establishment success and spread will be most visible for those alien species
that have characteristics favored by the changing climate, such as those that are drought tolerant or able to take
advantage of higher temperatures.

290
Chapter 4 Terrestrial and Inland Water Systems
4
b
ark beetle, and resulted in forest destruction (high confidence; Raffa
et al., 2008). The interactions between crop growth, climate change, and
pest or pathogen dynamics are difficult to predict (West et al., 2012).
Management strategies may become less effective as a consequence of
the decoupling of biocontrol relationships and less effective mechanical
control as biomass and/or population size of invasive species increases
(low to medium confidence; Hellmann et al., 2008).
4.3. Vulnerability of Terrestrial and Freshwater
Ecosystems to Climate Change
The vulnerability of ecosystems to climate change, that is, their propensity
to be adversely affected, is determined by the sensitivity of ecosystem
processes to the particular elements of climate undergoing change and
the degree to which the system (including its coupled social elements) can
maintain its structure, composition, and function in the presence of such
change, either by tolerating or adapting to it. Tolerance and adaptability
both interact with exposure, which in the case of terrestrial and freshwater
ecosystems means the magnitude and rate of climate change relative
to ranges of climatic conditions and rates of change under which the
ecosystem developed and its organisms evolved. Chapter 19 provides
a full discussion on vulnerability concepts.
4.3.1. Changes in the Disturbance Regime
The species composition at a given location is determined by three
considerations: the ability of species to reach the location; the physiological
tolerance of the species in relation to the range of conditions experienced
there; and interactions with other species, including competitors,
symbionts, predators, prey, and pathogens. Occasional disturbances
relieve competition, create opportunities for the establishment and
success of less dominant species, and may facilitate dispersal. Moderate
disturbance is thus important in maintaining diversity and ecosystem
function (Connell, 1978). Exposure to disturbances keeps tolerance of
disturbance in the population high. Fire, floods, and strong winds are
all examples of biodiversity-sustaining climate disturbances, provided
that their frequency and intensity do not deviate greatly above or below
the regime to which the species are adapted. Average environmental
conditions may be less of a determinant of species range and abundance
than the extreme conditions, such as the occurrence of exceptionally
cold or hot days or droughts exceeding a certain duration (Zimmermann
et al., 2009). The projected changes in probability of extremes are
typically disproportionately larger than the projected changes in
the mean (see IPCC, 2012; but also Diffenbaugh et al., 2005). Biotic
disturbances, such as pest and pathogen outbreaks are also often
implicated in ecosystem change, and may be enabled by climate change.
It is suggested that ecosystem regime shifts resulting from climate
change (alone or in interaction with other factors) will often be triggered
by changes in the disturbance regime, rather than by physiological
tolerance for the mean conditions (Thonicke et al., 2001). A “disturbance
regime” refers to the totality of different types of disturbance events in
a system, each characterized by its probability of occurrence, intensity,
and other relevant attributes, such as its seasonal pattern. A corollary
is that disturbance-related change is abrupt rather than gradual. Change
i
n the fire disturbance regime is emerging as a key proximal mechanism
and early indicator of terrestrial ecosystem change (Girardin et al., 2009;
Johnstone et al., 2010). Changes in the fire regime have in some cases
been attributed to climate change (Littell et al., 2009). Regional trends
in fire occurrence have been observed since 2000 (Giglio et al., 2013),
but interpreting their significance requires a longer term perspective
(e.g., Bergeron et al., 2010).
4.3.2. Observed and Projected Change in Ecosystems
This section highlights key observed changes in terrestrial and freshwater
ecosystems over the recent past, as well as changes projected during
the 21st century. For observations, we assess the degree of confidence
that change has been detected, and separately the confidence we have
in attributing the change to climate change (Figure 4-4). Confidence in
detection is considered to be very high when there is high agreement
between many independent studies, species, ecosystems, or regions
and where there is robust evidence that the changes over time are
statistically significant (see Chapter 18; Mastrandrea et al., 2010). Note
that a slightly different definition of detection is used here than in Chapter
18, because detection here is based solely on the presence of a temporal
trend and does not attempt to distinguish natural from climate-related
variation. Confidence in attribution to climate change is very high when
three tests are satisfied: changes correspond to a sound mechanistic
understanding of responses to climate change; the time series of
observations is sufficiently long to detect trends correlated with climate
change; and confounding factors can be accounted for or are of limited
importance. In the sections that provide the details of the assessment
of detection and attribution, estimated levels of confidence are given
even in cases where the capacity for detection or attribution capacity
is low or very low, because changes in these ecosystem properties or
processes could have large impacts on biodiversity or ecosystem services
at regional to global scales. In all cases the estimates of confidence levels
are based on global and cross-taxon assessments, so the positioning
may be different for specific taxa or regions. Some of the sections include
assessments of model-based projections of future change; the confidence
assessment of detection and attribution does not extend to these.
A key message arising from the analysis of detection and attribution is
that climate impacts on the functioning of organisms and ecosystems
are clearest when temperature is a principal driver, changes are relatively
rapid, and confounding factors play a small role. At one end of the
spectrum, the large warming signal over the last several decades in
much of the Arctic tundra combined with minimal human impacts is
associated with high confidence in detection of an increase in shrubs
and permafrost thawing and high confidence in the attribution to climate
warming (Section 4.3.3.1.1). Likewise, the phenology of most organisms
is sensitive to temperature, confounding effects are often small, and the
response is rapid, leading to high confidence in detection and attribution
of changes in phenology to warming (Section 4.3.2.1). At the opposite
end of the spectrum, species extinctions are very difficult to attribute to
climate change (Section 4.3.2.5), in part because other factors dominate
recent extinctions. This does not mean that climate has not played an
important contributing role; indeed it has been argued that the low
level of confidence in attribution is due to the lack of studies looking
for climate signals in extinctions (Cahill et al., 2013). Similarly there is
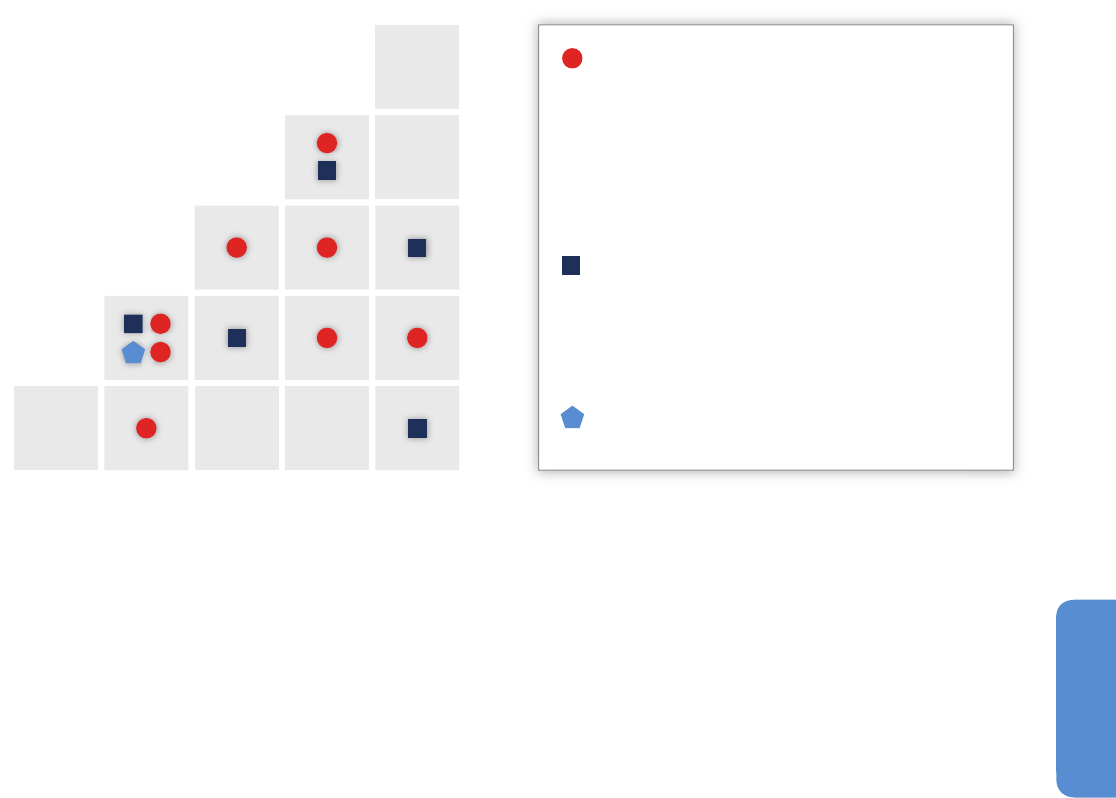
291
Terrestrial and Inland Water Systems Chapter 4
4
very good evidence that species composition is changing in cultural
landscapes, but the important role of other factors, for example, land
management and nitrogen deposition, makes attribution of a contribution
to recent warming difficult. This analysis indicates that responses in
most species and ecosystem levels will become more apparent over
time because (1) observed organism-level changes will have long-term
impacts on ecosystem functioning (high confidence; Sections 4.3.2.1,
4.3.2.5, 4.3.3) and (2) warming signals can be detected in ecosystems
where the recent warming has been strong and confounding factors
are minimal. In addition, the absence of observed changes does not
preclude confident projections of future change for three reasons: climate
change projected for the 21st century substantially exceeds the changes
experienced over the past century in medium to high scenarios (all but
RCP2.6); ecosystem responses to climate change may be nonlinear; and
change may be apparent only after considerable time lags (Jones et al.,
2009).
4.3.2.1. Phenology
Further evidence from ground-based and satellite studies, focused
mainly on the NH (Northern Hemisphere), supports the AR4 conclusion
that shifts in phenology have occurred over recent decades. “Spring
advancement”—earlier occurrence of spring events, such as breeding,
bud burst, breaking hibernation, flowering, migration—is seen in
hundreds of plant and animal species in many regions (Menzel et al.,
2006; Cleland et al., 2007; Parmesan, 2007; Primack et al., 2009; Cook
et al., 2012a; Peñuelas et al., 2013), although magnitudes of change
vary considerably and some species show no change (Parmesan, 2007).
Apparent discrepancies between two estimates of overall NH spring
advancement noted in AR4 (-2.3 days per decade, Parmesan and Yohe,
2003; -5.1 days per decade, Root et al., 2003) are largely resolved when
methodological differences are accounted for, particularly the inclusion
of species that do not show phenological changes (Parmesan, 2007). A
combined analysis of 203 species suggests NH spring advancement of
-2.8 ± 0.35 days per decade (Parmesan, 2007).
4.3.2.1.1. Plants
Spring advancement is seen across the NH including North America
(e.g., Cook et al., 2008, 2012b), Europe (e.g., Menzel et al., 2006; Cook
et al., 2012b), Asia (e.g., Primack et al., 2009; Ma and Zhou, 2012), and
the High Arctic (Høye et al., 2007). Changes are generally larger at higher
latitudes. A meta-analysis indicates mean NH spring advancement of
-1.1 ± 0.16 days per decade for herbs and grasses (85 species), -1.1 ±
0.68 days per decade for shrubs (6 species), and -3.3 ± 0.87 days per
decade for trees (16 species), over a record period of 35 to 132 years,
depending on the study. The warming trends detected in the well-mixed
surface waters (epilimnion) of many lakes in North America, Eurasia,
and Africa (Adrian et al., 2009) are associated with the earlier onset of
spring phytoplankton blooms (Winder and Schindler, 2004; Winder and
Sommer, 2012). Satellite data also indicate a general tendency of spring
advancement, though there is variation between satellite studies,
especially at local scales, due to the use of different instruments and
methods (e.g., White et al., 2009). A study using the Advanced Very High
Resolution Radiometer (AVHRR) suggests that for vegetation between
30ºN and 80ºN, the start of the growing season advanced by -5.2 days
Very low
Very low
Low Medium
Degree of confidence in detection
High Very high
Low Medium
Degree of confidence in attribution
High Very high
7
8
1
2
3
4
5
6
9
1
4
12
1
3
11
10
1. Changes in evapotranspiration (Section 4.3.2.4)
2. Increased tree mortality (Section 4.3.3.1, Box 4-2)
3. Increased extinctions (Section 4.3.2.5)
4. Increased primary productivity (Section 4.3.2.2) and carbon stocks
(Section 4.3.2.3)
5
. Changes in phenology (Section 4.3.2.1)
6. Species range shifts (Section 4.3.2.5)
7. Invasive species (Section 4.2.4.6)
8
. Flow-related impacts on freshwater ecosystems (Section 4.3.3.3)
9. Cultural landscapes – species composition changes (Section 4.3.3.5)
10. Tundra – increase in shrubs, melting of permafrost (Section 4.3.3.4,
Box 4-4)
11. Boreal – tree mortality (Section 4.3.3.1.1, Box 4-4)
12. Amazon – tree mortality (Section 4.3.3.1.3, Box 4-3)
13. Savannahs – woody encroachment (Section 4.3.3.2.2)
1
4. Evolutionary and genetic adaptation (Section 4.4.1)
Evidence of change in species and ecosystems
Impacts on major systems including early signs of regime shifts
Adaptation
Figure 4-4 | Confidence in detection of change and attribution of observed responses of terrestrial ecosystems to climate change. Confidence levels are based on expert
judgment of the available literature following the IPCC uncertainty guidance (Mastrandrea et al., 2010), attribution criteria outlined in Chapter 18, and detection criteria defined
in the text. The symbols in the figure represent global and cross-taxon assessments; the positioning may be different for specific taxa or regions. Details of the assessments that
were used in positioning each of the points can be found in the sections given in parentheses.

292
Chapter 4 Terrestrial and Inland Water Systems
4
b
etween 1999 and 1982 and advanced a further -0.2 days by 2008;
while the growing season end was delayed by 6.6 days between 1982
and 2008 (Jeong et al., 2011). Studies with a more recent satellite
instrument, the Moderate Resolution Imaging Spectrometer (MODIS),
also show spring advancement (e.g., Ahl et al., 2006). The relatively short
duration of satellite observations makes trend detection particularly
sensitive to the choice of analysis period.
4.3.2.1.2. Animals
Many new studies provide further evidence of changes in animal
phenology (e.g., amphibians: Kusano and Inoue, 2008; Phillimore et al.,
2010; birds: Pulido, 2007; Thorup et al., 2007; mammals: Adamik and Kral,
2008; Lane et al., 2012; insects: Robinet and Roques, 2010; freshwater
plankton: Adrian et al., 2009). Changes in breeding phenology are
reported from various regions and different taxa (e.g., Parmesan, 2006,
2007; Post et al., 2008; Primack et al., 2009). In the NH several studies
show advancements of egg laying dates in birds (e.g., Parmesan, 2007:
-3.7 ± 0.7 days per decade, in 41 species). In contrast, a delay of the
mean breeding date by 2.8 to 3.7 days between 1950 and 2004 was seen
for two of nine seabirds in the Eastern Antarctic, linked to decreased sea
ice extent (Barbraud and Weimerskirch, 2006). Spring arrival dates have
advanced for many migratory birds (e.g., Thorup et al., 2007). Patterns
of changes in autumn migration in birds are mostly not consistent
(delayed, advanced, no change) across analyzed species and regions
and appear to be highly related to non-climatic variables (e.g., Sokolov,
2006; Adamik and Pietruszkova, 2008).
A large body of evidence therefore shows that, in NH temperate, boreal,
and Arctic regions, spring advancement has occurred in many plant and
animal species over the last several decades (high confidence due to
robust evidence but only medium agreement when examined across all
species and regions; Figure 4-4).
Understanding of the drivers of phenological change has also improved
further since AR4. Many observational studies find a correlation with
higher temperatures (Cook et al., 2012a). Experimental manipulation
generally supports this (e.g., plants: Cleland et al., 2012; bird egg-laying:
Visser et al., 2009; insects: Musolin et al., 2010; Kollberg et al., 2013).
Some individual studies find good agreement between experimental
warming and in situ observations (e.g., Gunderson et al., 2012) although
a meta-analysis suggests that experiments can substantially under-
predictadvances in the timing of flowering and leafing of plants in
comparison with observational studies (Wolkovich et al., 2012).
Observational data can also be affected by methodological issues; for
example, flipper-tagging of penguins can alter their migratory behavior
(Saraux et al., 2011). Rates of warming across a season may also be
important (Schaper et al., 2012). Models can be used to explain
relationships between observed phenological changes and environmental
variables. For example, a model based on water temperature captured
the observed temporal and spatial variation in Daphnia phenology in
NH lakes (Straile et al., 2012). Other environmental factors related to
temperature, such as timing of snowmelt, snow cover, and snow depth,
can play a role. Snowmelt changes led to earlier flowering and appearances
of plants and arthropods in Greenland between 1996 and 2005 (Høye et
al., 2007) and earlier flowering in an alpine plant in the Rocky Mountains,
U
SA, between 1975 and 2008 (Hülber et al., 2010; Lambert et al., 2010).
Earlier snowmelts decreased floral resources and hence affected insect
population dynamics in mountain ranges in the USA in the years 1980,
1985, 1986, and 1989 (Boggs and Inouye, 2012). In Colorado, USA, the
yellow-bellied marmot emerged earlier from hibernation due to
snowmelts becoming earlier over 1976–2008 (Ozgul et al., 2010) while
in Alberta, Canada, Columbian ground squirrels emerged later over
1992–2012 owing to delayed snowmelts associated with increased
late-season snowstorms (Lane et al., 2012). Delayed emergence from
hibernation was associated with decreased population growth rate
(Lane et al., 2012). Food availability can be important; for example, in
the Yukon area, Canada, the date of giving birth in North American
squirrels (Tamiascurus hudsonicus) advanced by an average of -18 days
over the period 1989–1998, coinciding with increasing abundance of
white spruce cones, their major food source (Réale et al., 2003).
Phenological response can differ with migration strategy in birds, for
example short-distance migrants show greater advancements in spring
arrivals than long distant migrants (e.g., Saino et al., 2009; but see
Parmesan, 2006 for different patterns). In a temperate region
(Massachusetts, USA), declining sizes of populations and migrating
cohorts of North American Passerine birds account for a large part of
the variation in migration times between 1970 and 2002 (Miller-Rushing
et al., 2008). The remaining variation was explained by climatic variables,
migration distance, and date. The variation in bird migration phenology
change can also be related to differing patterns of feather changes during
moulting times, food availability at stop-over places, and differing health
conditions of individual species (Gordo, 2007).
Although a number of non-climatic influences on phenology are also
identified, an increased number of observational and experimental studies,
across many organism types, suggest that warming has contributed to
the overall spring advancement observed in the NH (high confidence due
to high agreement and medium evidence).
4.3.2.2. Primary Productivity
Primary production, the process of plant growth, is fundamental to the
global carbon cycle (see Section 4.3.2.3) and underpins provisioning
ecosystem services such as food, timber, and grazing. Trends in the
amount, seasonal timing, variability, location, and type of primary
production are therefore important indicators of ecosystem function.
Well-established theory, experimentation, and observation all agree that
primary production is directly sensitive to most aspects of climate change,
is indirectly affected via the effects of climate on pests and diseases,
and is responsive to many of the other changes simultaneously taking
place in the world, such as described in Section 4.2.4. The diverse and
frequently nonlinear form of responses to the factors influencing
primary production, combined with the complexity of interactions
between them, means that at a given location the net outcome can be
an increase, no change, or a decrease in productivity.
The concentration of CO
2
in the atmosphere shows clear patterns in
space and time largely related to the primary productivity of the land
and oceans. The contribution by terrestrial ecosystems to these patterns
can be estimated using isotope measurements, emission databases, and

293
Terrestrial and Inland Water Systems Chapter 4
4
m
odels (Canadell et al., 2007). It consists of a sink term, due to increased
net ecosystem production, plus a source term due to land use change.
During the decade 2000–2009, land net primary productivity at the
global scale continued to be enhanced about 5% relative to the estimated
preindustrial level, leading to a land sink of 2.6 + 1.2 PgC yr
–1
(these
values are from WGI AR5 Section 6.3.2.6; the uncertainty range is 2
standard deviations; for the primary literature see also Raupach et al.,
2008; Le Quéré et al., 2009). The net uptake of carbon by the land is
highly variable year to year, mainly in response to climate variation and
major volcanic eruptions (Peylin et al., 2005; Sitch et al., 2008; Mercado
et al., 2009). Given the uncertainty range, it is not possible to conclude
whether the rate of carbon uptake by the residual land sink has
increased or decreased over the past 2 decades (Raupach et al., 2008;
WGI AR5 Section 6.3.2.6). Coupled Model Intercomparison Project Phase
5 (CMIP5) model projections, using the RCP scenarios, suggest that the
rate of net carbon uptake by terrestrial ecosystems will decrease during
the 21st century except under the RCP4.5 scenario, and by the greatest
amount under RCP8.5. There is greater uncertainty between models
than between scenarios; in some models terrestrial ecosystems become
a net source of CO
2
to the atmosphere (WGI AR5 Section 6.4.3.2,
especially Figure 6.26).
It is possible to downscale the land sink estimate continentally, using
inversion modeling techniques and the growing network of precision
atmospheric observations. There is high agreement and medium evidence
that the net land uptake in natural and semi-natural terrestrial ecosystems
is broadly distributed around the world, almost equally between
forested and non-forested ecosystems, but is offset in the tropics by a
large carbon emission flux resulting from land use change, principally
deforestation (Pan et al., 2011).
The observed trends in Normalized Difference Vegetation Index (NDVI),
a satellite proxy for primary productivity, are discussed under various
ecosystem-specific discussions above and below. In some cases the
trends are sufficiently strong and consistent to support a confident
statement about the underlying phenomenon, but in many cases they
are not. This may mean that no change has occurred, or simply reflect
inadequacies in the indicator, method of analysis, and length of the
record in relation to the high interannual variability. AR4 reported a
trend of increasing seasonally accumulated NDVI (“greening”) at high
northern latitudes (Fischlin et al., 2007; based on Sitch et al., 2007),
but subsequent observations show a lower rate and no geographical
uniformity (Goetz et al., 2007). More than 25% of high-latitude North
American forest areas, excluding areas recently disturbed by fire,
showed a decline in greenness and no systematic change in growing
season length, particularly after 2000 (Goetz et al., 2007). NDVI trend
analyses in rangelands show varying patterns around the world, with
substantial disagreement between studies (Millennium Ecosystem
Assessment, 2005a; Bai et al., 2008; Beck, H.E. et al., 2011; Fensholt et
al., 2012). There is agreement that the Sahel showed widespread NDVI
increase between the mid-1980s and about 2000, along with an
increase in rainfall, but no consensus on whether the detected signal
represents increased productivity by grasses, trees, or herbs; and to what
degree it reveals land management efforts or responses to climate
(Anyamba and Tucker, 2005; Prince et al., 2007; Hellden and Tottrup,
2008; Seaquist et al., 2009). In the period 2000–2009 no NDVI trend
was apparent in the Sahel (Samanta et al., 2011).
T
ree rings record changes in tree growth over approximately the past
millennium. Many tree ring records show accelerated tree growth during
much of the 20th century (Briffa et al., 2008), which often correlates with
rising temperature. Variations in tree ring width, density, and isotopic
composition arise from many factors, including temperature, moisture
stress, CO
2
fertilization, N deposition, and O
3
damage, but also stand
structure and management. Direct CO
2
effects, inferred from the
ring record once the effects of drought and temperature have been
accounted for, have been proposed for approximately 20% of the sites
in the International Tree Ring Data Base (Gedalof and Berg, 2010) and
studied in detail at some sites (Koutavas, 2008). Since the 1980s, a
number of tree ring records show a decline in tree growth (Wilson et al.,
2007). Several possible causes have been suggested for this, including
increasing water stress and O
3
damage; but the most recent rings in
most published tree ring chronologies date from before the 1990s
(Gedalof and Berg, 2010), so tree ring-based conclusions for the past
2 decades are based on a relatively small body of evidence and may
therefore be biased. Recent tree ring studies were often specifically
designed to examine growth in response to environmental changes
(Gedalof and Berg, 2010) and may therefore not be representative of
global tree growth. Direct repeated measurements of tree girth increment
in forest monitoring plots (discussed in Section 4.3.2.3) are an alternate
data source for recent decades.
Primary production in freshwater lakes has been observed to increase
in some Arctic (Michelutti et al., 2005) and boreal lakes, but to decrease
in Lake Tanganyika in the tropics (O’Reilly et al., 2003). In both cases
the changes were attributed by the authors to climate change.
In summary, there is high confidence that net terrestrial ecosystem
productivity at the global scale has increased relative to the preindustrial
era. There is low confidence in attribution of these trends to climate change.
Most studies speculate that rising CO
2
concentrations are contributing
to this trend through stimulation of photosynthesis, but there is no clear,
consistent signal of a climate change contribution (Figure 4-4).
4.3.2.3. Biomass and Carbon Stocks
The forest biomass carbon stock can be estimated from the routine forest
monitoring that takes place for management and research purposes.
Forest inventories were generally designed to track timber volumes;
inferring total biomass and ecosystem carbon stocks requires further
information and assumptions, which make absolute values less certain,
but have a lesser effect on trend detection. Forest inventory systems are
well developed for NH temperate and boreal forest (Nabuurs et al., 2010;
Ryan et al., 2010; Wang, B. et al., 2010). Data for tropical and Southern
Hemisphere forests and woodlands also exist (Maniatis et al., 2011;
Tomppo et al., 2010) but are typically less available and comprehensive
(Romijn et al., 2012). More and better data may become available as a
result of advances in remote sensing (e.g., Baccini et al., 2012) and
increased investment in forest monitoring through initiatives such as the
Reduced Emissions from Deforestation and Degradation (REDD) of the
United Nations Framework Convention on Climate Change (UNFCCC).
Forests have increased in biomass and carbon stocks over the past half
century in Europe (Ciais et al., 2008; Luyssaert et al., 2010) and the USA

294
Chapter 4 Terrestrial and Inland Water Systems
4
(
Birdsey et al., 2006). Canadian managed forests increased in biomass
only slightly during 1998–2008, because growth was offset by significant
losses due to fires and beetle outbreaks (Stinson et al., 2011). Several
dozen sites across the moist tropics have been monitored to estimate
forest biomass changes. In the Amazon (Phillips et al., 2009) forest
biomass has generally increased in recent decades, dropping temporarily
after a drought in 2005. Globally, for the period 2000–2007, recently
undisturbed forests are estimated to have withdrawn 2.30 ± 0.49 PgC
yr
–1
from the atmosphere, while formerly cleared tropical forests, now
regrowing, withdrew an additional 1.72 ± 0.54 PgC yr
–1
(Pan et al.,
2011). The global terrestrial carbon sink is partly offset by the losses of
forest carbon stocks to the atmosphere through land use change, largely
in the tropics, of 1.1 ± 0.8 PgC yr
–1
(2000–2009, WGI AR5 Section 6.3.2.6).
The carbon stock in global soils, including litter and peatlands is 1500
to 2400 PgC, with permanently frozen soils adding another 1700 PgC
(Davidson and Janssens, 2006). The soil carbon stock is thus more than
10 times greater than the carbon stock in forest biomass (Kindermann
et al., 2008). Changes in the size of the soil carbon stock result from
changes in the net balance of inputs and losses over a period of many
years. Inputs derive from primary production, discussed in Section 4.3.2.2,
and are mostly modestly increasing under climate change. Losses result
principally through the respiration of soil microbes, which increases with
increasing temperature. The present and future temperature sensitivity
of microbial respiration remains uncertain (Davidson and Janssens,
2006). An analysis of long-term respiration measurements from the soil
around the world suggests that it has increased over the past 2 decades
by an amount of 0.1 PgC yr
–1
, some of which may be due to increased
productivity (Bond-Lamberty and Thomson, 2010). If soil respiration were
to exceed terrestrial net primary production globally and on a sustained
basis, the present net terrestrial sink would become a net source,
accelerating the rate of CO
2
build-up in the atmosphere (Luo, 2007).
The carbon stock in freshwater systems is also quite high in global terms.
Annual rates of storage (0.03 to 0.07 PgC yr
–1
) may be trivial compared
with sequestration by soils and terrestrial vegetation, but lake sediments
are preserved over longer time scales (+10 kyr compared with decades
to centuries), and Holocene storage of carbon in lake sediments has
been estimated at 820 Pg (Cole et al., 2007). Manmade impoundments
represent an increasing and short-lived additional carbon store with
conservative annual estimates of 0.16 to 0.2 PgC yr
–1
(Cole et al., 2007).
A short-duration study of the temperature sensitivity of decomposition
in flooded coastal soils, extrapolated to the 21st century, suggested that
increases in respiration would exceed increases in future production
(Kirwan and Blum, 2011). Further detail on wetland soil carbon stocks
can be found in Section 4.3.3.3 on peatlands and on permafrost carbon
stocks in Box 4-4 and in Chapter 28.
In summary, biomass and soil carbon stocks in terrestrial ecosystems
are currently increasing (high confidence) but are vulnerable to loss to
the atmosphere as a result of rising temperature, drought, and fire
projected in the 21st century (Figure 4-4). Measurements of increased
tree growth over the last several decades, a large sink for carbon, are
consistent with this but confounding factors such as N deposition,
afforestation, and land management make attribution of these trends
to climate change difficult (low confidence).
4.3.2.4. Evapotranspiration and Water Use Efficiency
Evapotranspiration (ET) includes evaporation from the ground and
vegetation surfaces, and transpiration through plant stomata. Both are
affected by multiple factors (Luo et al., 2008) including temperature,
solar (shortwave) and thermal (longwave) radiation, humidity, soil
moisture, and terrestrial water storage; transpiration is additionally
affected by CO
2
concentration through its influence on plant stomatal
conductance. Studies using lysimeters, evaporation pans, the balance
of observed precipitation and runoff, and model reconstructions indicate
both increases and decreases in ET in different regions and between
approximately 1950 and the present (Huntington, 2008; Teuling et al.,
2009; Douville et al., 2013). Flux tower records have at most 15 years
duration (FLUXNET, 2012), so there are insufficient data to calculate large-
scale, long-term trends. ET can also be estimated from meteorological
observations or simulated with models constrained by observations.
Estimates of ET from 1120 globally (but non-uniformly) distributed
stations indicate that global land mean ET increased by approximately
2.2% between 1982 and 2002, a rate of increase of 0.75 mm yr
–2
(Wang, K. et al., 2010). Other studies, using data-constrained models,
indicated global ET rises of between 0.25 and 1.1 mm yr
–
2
during the
1980s and 1990s (Jung et al., 2010; Vinukollu et al., 2011; Zeng et al.,
2012), possibly linked with increased surface solar radiation and
thermal radiation (Wild et al., 2008) or warming (Jung et al., 2010).
There has been no significant ET trend since approximately 2000
(Jung et al., 2010; Vinukollu et al., 2011; Zeng et al., 2012), possibly due
to soil moisture limitation (Jung et al., 2010). Overall, there is low
confidence in both detection and attribution of long-term trends in ET
(Figure 4-4).
Experiments show that rising CO
2
decreases transpiration and increases
intrinsic water use efficiency (iWUE, the ratio of photosynthesis to
stomatal conductance; Leakey et al., 2009). Some modeling studies
suggest that, over the 20th century, the effects of CO
2
on decreasing
transpiration are of comparable size but opposite to the effects of rising
temperature (Gerten et al., 2008; Peng et al., 2013). However, the
observed general increase in ET argues that reduced transpiration cannot
be the dominant factor (Huntington, 2008). A meta-analysis of studies
at 47 sites across five ecosystem types (Peñuelas et al., 2011) suggests
that iWUE for mature trees increased by 20.5% between the 1970s and
2000s. Increased iWUE since preindustrial times (1850 or before) has
also been found at several forest sites (Andreu-Hayles et al., 2011;
Gagen et al., 2011; Loader et al., 2011; Nock et al., 2011) and also in a
temperate semi-natural grassland since 1857 (Koehler et al., 2010),
although in one boreal tree species iWUE ceased to increase after 1970
(Gagen et al., 2011).
4.3.2.5. Changes in Species Range, Abundance, and Extinction
Species respond to climate change through genotypic adaptation and
phenotypic plasticity; by moving out of unfavorable and into favorable
climates; or by going locally or globally extinct (Dawson et al., 2011;
Bellard et al., 2012; Peñuelas et al., 2013; see also Section 4.2.3). These
responses to climate change can potentially have large impacts on
biodiversity and ecosystem services. Genotypic adaptation in the face of
strong selection pressure from climate change is typically accompanied

295
Terrestrial and Inland Water Systems Chapter 4
4
by large reductions in abundance (see Section 4.4.1.2). Species range
shifts are accompanied by changes in abundance, local extinctions, and
colonization that can alter ecosystem services when they affect dominant
species such as trees, keystone species such as pollinators, or species
that are vectors for diseases (Zarnetske et al., 2012). Global extinctions
result in the permanent loss of unique forms of life.
Substantial evidence has accumulated since AR4 reinforcing the conclusion
that the geographical ranges of many terrestrial and freshwater plant
and animal species have moved over the last several decades in response
to warming and that this movement is projected to accelerate over the
coming decades under high rates of climate change. Some changes in
species abundances appear to be linked to climate change in a predictable
manner, with species abundances increasing in areas where climate has
become more favorable and vice versa. In contrast, uncertainties
concerning attribution to climate change of recent global species
extinctions, and in projections of future extinctions, have become more
apparent since the AR4.
4.3.2.5.1. Observed species range shifts
The number of studies looking at observed range shifts and the breadth
of species examined have greatly increased since AR4. The most
important advances since AR4 concern improvements in understanding
the relationship between range shifts and changes in climate over the
last several decades. The “uphill and poleward” view of species range shifts
in response to recent warming (Parmesan and Yohe, 2003; Parmesan,
2006; Fischlin et al., 2007; Chen et al., 2011) is a useful simplification
of species responses; however, responses to warming are conditioned
by changes in precipitation, land use, species interactions, and many
other factors. Investigations of the mechanisms underlying observed
range shifts show that climate signals can often be detected, but the
impacts of and interactions between changing temperature, precipitation,
and land use often result in range shifts that are downhill or away from
the poles (Rowe et al., 2010; Crimmins et al., 2011; Hockey et al., 2011;
McCain and Colwell, 2011; Rubidge et al., 2011; Pauli et al., 2012; Tingley
et al., 2012; Zhu et al., 2012). There are large differences in the ability
of species groups (i.e., broad taxonomic categories of species) and
species within these groups to track changes in climate through range
shifts (Angert et al., 2011; Mattila et al., 2011; Chen et al., 2011). For
example, butterflies appear to be able track climate better than birds
(community shifts: Devictor et al., 2012; but see Chen et al., 2011 for
range shifts) while some plants appear to be lagging far behind climate
trends except in mountainous areas (Bertrand et al., 2011; Doxford and
Freckleton, 2012; Gottfried et al., 2012; Zhu et al., 2012; Telwala et al.,
2013). There is growing evidence that responses at the “trailing edge”
of species distributions (i.e., local extinction in areas where climate has
become unfavorable) are often less pronounced than responses at the
“leading edge” (i.e., colonization of areas where climate has become
favorable), which may be related to differences in the rates of local
extinction vs. colonization processes (Doak and Morris, 2010; Chen et
al., 2011; Brommer et al., 2012; Sunday et al., 2012) and difficulties in
detecting local extinction with confidence (Thomas et al., 2006).
Rising water temperatures are also implicated in species range shifts
in river fish communities (e.g., Comte and Grenouillet, 2013), combined
with a decrease in recruitment and survival as well as range contraction
of cold-water species such as salmonids (Bartholow, 2005; Bryant, 2009;
Ficke et al., 2007; Jonsson and Jonsson, 2009; Hague et al., 2011). Shifts
in freshwater fish species range toward higher elevation and upstream
(Hickling et al., 2006; Comte and Grenouillet, 2013) also are not keeping
pace with the rate of warming in streams and rivers. While these
changes in river temperature regimes may also open up new habitat at
higher latitudes (or altitudes) for migratory (Reist et al., 2006) and cool-
and warm-water species of fish (Tisseuil et al., 2012), there is high
confidence that range contraction threatens the long-term persistence
of some fully aquatic species.
Rates of recent climate change have varied greatly across the globe,
ranging from rapid warming to cooling (Burrows et al., 2011; Dobrowski
et al., 2013). Taking this spatial variation into account should enhance
the ability to detect climate-related range shifts. A recent synthesis of
range shifts indicates that terrestrial animal species have moved at rates
that correspond better with changes in temperature when climate is
measured only in the regions where the range shifts were observed
Frequently Asked Questions
FAQ 4.4 | How does climate change contribute to species extinction?
There is a consensus that climate change over the coming century will increase the risk of extinction for many species.
When a species becomes extinct, a unique and irreplaceable life form is lost. Even local extinctions can impair the
healthy functioning of ecosystems.
Under the fastest rates and largest amounts of projected climate change, many species will be unable to move fast
enough to track suitable environments, which will greatly reduce their chances of survival. Under the lowest projected
rates and amounts of climate change, and with the assistance of effective conservation actions, the large majority of
species will be able to adapt to new climates, or move to places that improve their chances of survival. Loss of habitat
and the presence of barriers to species movement increase the risk of extinctions as a result of climate change.
Climate change may have already contributed to the extinction of a small number of species, such as frogs and toads
in Central America, but the role of climate change in these recent extinctions is the subject of considerable debate.

296
Chapter 4 Terrestrial and Inland Water Systems
4
(
Chen et al., 2011), providing greater confidence in attribution of the
range shifts to climate change. Average range shifts across taxa and
regions in this study were approximately 17 km poleward and 11 m up
in altitude per decade, velocities that are two to three times greater than
previous estimates (compare with Parmesan and Yohe, 2003; Fischlin et
al., 2007), but these responses differ greatly among species groups.
However, this approach remains a simplification, as the climate drivers
of species range changes, for example, temperature and precipitation,
have frequently shifted in different geographical directions (Dobrowski
et al., 2013). Disentangling these conflicting climate signals can help
explain complex responses of species ranges to changes in climate
(Tingley et al., 2012). Overall, studies since AR4 show that species range
changes result from interactions among climate drivers and between
climate and non-climate factors. It is the greater understanding of these
interactions, combined with increased geographical scope, that leads
to high confidence that several well-studied species groups, such as
insects and birds, have shifted their ranges over significant distances
(tens of kilometers or more) over the last several decades, and that these
range shifts can be attributed to changes in climate. But for many other
species groups range shifts are more difficult to attribute to changes in
climate because the climate signal is small, there are many confounding
factors, differences between expected and observed range shifts are
large, or variability within or between studies is high. Thus there is only
medium confidence in detection and attribution when examined across
all species and all regions.
4.3.2.5.2. Future range shifts
Projections of climate change impacts on future species range shifts
since the AR4 have been dominated by studies using Ecological Niche
Models (ENMs) that project future ranges based on correlative models
of current relationships between environmental factors and species
distribution (Peterson et al., 2011). A variety of process-based models
are starting to be more widely used to make projections of future species
distributions (Buckley et al., 2010; Beale and Lennon, 2012; Cheaib et
al., 2012; Higgins et al., 2012; Foden et al., 2013). Model comparisons
show that correlative models generally predict larger range shifts than
process-based models for trees (Morin and Thuiller, 2009; Kearney et
al., 2010; Cheaib et al., 2012). For other species groups that have been
studied, differences in projections between model types show no clear
tendency (Kearney et al., 2009; Buckley et al., 2010; Bateman et al.,
2012). There has been some progress in model validation: projected
species shifts are broadly coherent with species responses to climate
change in the paleontological record and with observed recent species
shifts (see Section 4.2.2 and above in this section), but further validation
is needed (Green et al., 2008; Pearman et al., 2008; Nogues-Bravo et al.,
2010; Dawson et al., 2011). Modeling studies typically do not account
for a number of key mechanisms mediating range shifts, such as genetic
adaptation and phenotypic plasticity (see Section 4.4.1.2), species
interactions, or human-mediated effects. An important limitation in most
studies is that realistic species displacement rates are not accounted
for (i.e., rates at which species are able to shift their ranges through
dispersal and establishment); as such, they only indicate changes in the
location of favorable and unfavorable climates, from which potential
shifts in species distribution can be inferred, but not rates of change
(Bateman et al., 2013).
A
nalyses and models developed since AR4 permit the estimation of the
ability of a wide range of species to track climate change. Figure 4-5
provides a synthesis of the projected abilities of several species groups
to track climate change. This analysis is based on (1) past and future
climate velocity, which is a measure of the rate of climate displacement
across a landscape and provides an indication of the speed at which an
organism would need to move in order to keep pace with the changing
climatic conditions (Loarie et al., 2009; Burrows et al., 2011; Chen et
al., 2011; Sandel et al., 2011; Feeley and Rehm, 2012; Dobrowski et al.,
2013); and (2) species displacement rates across landscapes for a broad
range of species (e.g., Stevens, V.M. et al., 2010; Nathan et al., 2011;
Barbet-Massin et al., 2012; Kappes and Haase, 2012; Meier et al., 2012;
Schloss et al., 2012; see additional references in Figure 4-5 legend).
Comparisons of these rates indicate whether species are projected to
be able to track climate as it changes. When species displacement
capacity exceeds climate velocity it is inferred that species will be able
to keep pace with climate change; when displacement capacity is lower
than projected climate velocities then they will not, within the bounds
of uncertainty of both parameters. This simplified analysis is coherent
with more sophisticated model analyses of climate-induced species
displacement across landscapes, some of which have evaluated additional
constraints such as demographics, habitat fragmentation, or competition
(e.g., Meier et al., 2012; Schloss et al., 2012).
Rates of climate change over the 20th century and projected for the
21st century are shown in Figure 4-5a. Rates of climate change for
global land surfaces are given for IPCC AR5 climate projections under
a wide range of GHG emissions scenarios (i.e., WGI AR5 Chapter 12;
Knutti and Sedláček, 2012). Rates of global warming for land surfaces
have averaged approximately 0.03°C yr
–1
since 1980, but have slowed
over the last decade and a half (WGI AR5 Chapter 2). At the low end of
projected future rates of warming, rates decrease over time, reaching
near zero by the end of the century (RCP2.6). At the high end, projected
rates increase over time, exceeding 0.06°C yr
–1
by the end of the century
(RCP8.5), and perhaps above 0.08°C yr
–1
at the upper bound.
Climate velocity is defined as the rate of change in climate over time
(e.g., °C yr
–1
, if only temperature is considered) divided by the rate of
change in climate over distance (e.g., °C km
–1
, if only temperature is
considered) and therefore depends on regional rates of climate change
and the degree of altitudinal relief (Figure 4-5b; Loarie et al., 2009;
Dobrowski et al., 2013). For example, climate velocity for temperature
is low in mountainous areas because the change in temperature over
short distances is large (e.g., Rocky Mountains, Andes, Alps, Himalayas;
Figure 4-5b, leftmost axis). Climate velocity for temperature is generally
high in flat areas because the rate of change in temperature over
distance is low (e.g., parts of the USA Midwest, Amazon basin, West
Africa, central Australia; Figure 4-5b, rightmost axis). In flat areas, climate
velocity can exceed 8 km yr
–1
for the highest rates of projected climate
change (RCP8.5). We have focused on climate velocity for temperature
change, but several analyses also account for precipitation change.
Rates of displacement vary greatly within and among species groups
(Figure 4-5c). Some species groups, notably herbaceous plants and trees,
generally have very low displacement capacity. Other species groups
such as butterflies, birds (not shown), and large vertebrates generally
have a very high capacity to disperse across landscapes, nonetheless
297
Terrestrial and Inland Water Systems Chapter 4
4
0246810
0246810
(a) Climate change scenarios
(km yr
–1
)
(km yr
–1
)
Rate of climate change for global land and freshwater areas (°C yr
–1
)
(°C yr
–1
)
Conversion
= 110 km°C
−1
Flat areas
30 km °C
−1
Global
a
verage
3.8 km°C
−1
Mountain
a
reas
Observed
Historical
RCP2.6 (+1.0°C)
RCP2.6-low
RCP8.5-high
RCP4.5 (+1.8°C)
RCP6.0 (+2.2°C)
RCP8.5 (+3.7°C)
−0.04
−0.02
0.00
0.02
0.04
0.06
0.08
0.00
0.02
0
.04
0.06
0.08
0.10
0.10
0.00
0.02
0
.04
0.06
0.08
0.10
1900 1950 2000 2050
Historical
Projected
2100
Lower
bound
Upper
bound
Projected mean
Estimated speed at which
species group can move
Median
Unable to keep up Able to keep up
(c) Species displacement rates (required to track climate velocity)
(a) Rate of climate change
(c) Species displacement rates
(b) Estimate of climate velocity to determine rate of displacement
Rate of temperature change
under RCP8.5 scenario
between 2050 and 2100
(Mean projected increase in global temperature for the period 2081–2100
(
WGI, Chapter 12))
0246810
0246810
0246810
0
0
0
0
0
0
0
0
0
0
0
0
0
Trees
Herbaceous
plants
Split-hoofed
mammals
Carnivorous
mammals
Rodents
Primates
Plant-feeding
insects
Freshwater
molluscs
Mountain areas
Global average
Flat areas
Figure 4-5 | (a) Rates of climate change, (b) corresponding climate velocities, and (c) rates of displacement of several terrestrial and freshwater species groups in the absence of
human intervention. Horizontal and vertical pink bands illustrate the interpretation of this figure. Climate velocities for a given range of rates of climate change are determined by
tracing a band from the range of rates in (a) to the points of intersection with the three climate velocity scalars in (b). Comparisons with species displacement rates are made by
tracing vertical bands from the points of intersection on the climate velocity scalars down to the species displacement rates in (c). Species groups with displacement rates below
the band are projected to be unable to track climate in the absence of human intervention. (a) Observed rates of climate change for global land areas are derived from Climatic
Research Unit/Hadley Centre gridded land-surface air temperature version 4 (CRUTEM4) climate data reanalysis; all other rates are calculated based on the average of Coupled
Model Intercomparison Project Phase 5 (CMIP5) climate model ensembles for the historical period (gray shading indicates model uncertainty) and for the future based on the four
Representative Concentration Pathway (RCP) emissions scenarios. Data were smoothed using a 20-year sliding window, and rates are means of between 17 and 30 models using
one member per model. Global average temperatures at the end of the 21st century for the four RCP scenarios are from WGI AR5 Chapter 12. (b) Estimates of climate velocity for
temperature were synthesized from historical and projected future relationships between rates of temperature change and climate velocity (historical: Burrows et al., 2011; Chen
et al., 2011; Dobrowski et al., 2013; projected future: Loarie et al., 2009; Sandel et al., 2011; Feeley and Rehm, 2012). The three scalars are climate velocities that are
representative of mountainous areas (left), averaged across global land areas (center), and large flat regions (right). (c) Rates of displacement are given with an estimate of the
median (black bars) and range (boxes = approximately 95% of observations or models for herbaceous plants, trees, and plant-feeding insects or median ± 1.5 inter-quartile
range for mammals). Displacement rates for herbaceous plants were derived from paleobotanical records, modern plant invasion rates, and genetic analyses (Kinlan and Gaines,
2003). Displacement estimates for trees are based on reconstructed rates of tree migration during the Holocene (Clark, 1998; Clark et al., 2003; Kinlan and Gaines, 2003;
McLachlan et al., 2005; Nathan, 2006; Pearson, 2006) and modeled tree dispersal and establishment in response to future climate change (Higgins et al., 2003; Iverson et al.,
2004; Epstein et al., 2007; Goetz et al., 2011; Nathan et al., 2011; Meier et al., 2012; Sato and Ise, 2012). Displacement rates for mammals were based on modeled dispersal
rates of a wide range of mammal species (mean of Schloss et al., 2012 for Western Hemisphere mammals and rates calculated from global assessments of dispersal distance by
Santini et al., 2013 and generation length by Pacifici et al., 2013). Displacement rates for phytophagous insects are based on observed dispersal distances and genetic analyses
(Peterson and Denno, 1998; Kinlan and Gaines, 2003; Schneider, 2003; Berg et al., 2010; Chen et al., 2011). The estimate of median displacement rate for this group exceeds the
highest rates on the axis. These displacement rates do not take into account limitations imposed by host plants. Displacement estimates for freshwater molluscs correspond to the
range of passive plus active dispersal rates for upstream movement (Kappes and Haase, 2012).

298
Chapter 4 Terrestrial and Inland Water Systems
4
s
ome species in these groups have low dispersal capacity. Current and
future rates of climate change correspond to climate velocities that exceed
rates of displacement for several species groups for most climate
change scenarios. This is particularly true for mid- and late-successional
trees that have maximum displacement rates that are on the order of
tens to a few hundreds of meters per year. Overall, many plant species
are foreseen to be able to track climates only in mountainous areas at
medium to high rates of warming, though there is uncertainty concerning
the potential role of long-distance dispersal (Pearson, 2006). Primates
generally have substantially higher dispersal capacity than trees;
however, a large fraction of primates are found in regions with very
high climate velocities, in particular the Amazon basin, thereby putting
them at high risk of being unable to track climates even at relatively
low rates of climate change (Schloss et al., 2012). On a global average,
many rodents, as well as some carnivores and freshwater molluscs, are
projected to be unable to track climate at very high rates of climate
change (i.e., >0.06°C yr
–1
). These projected differences in species ability
to keep pace with future climate change are broadly coherent with
observations of species ability or inability to track recent global warming
(see Section 4.3.2.5.1).
Humans can increase species displacement rates by intentionally or
unintentionally dispersing individuals or propagules. For example, many
economically important tree species may be deliberately moved on large
scales as part of climate adaptation strategies in forestry in some regions
(Lindner et al., 2010). Human activities can also substantially reduce
displacement rates. In particular, habitat loss and fragmentation typically
reduces displacement rates, sometimes substantially (Eycott et al., 2012;
Hodgson et al., 2012; Meier et al., 2012; Schloss et al., 2012). The degree
to which habitat fragmentation slows displacement depends on many
factors, including the spatial pattern of the fragments and corridors,
maximum dispersal distances, population dynamics, and the suitability of
intervening modified habitats as stepping-stones (Pearson and Dawson,
2003). Species and habitat dependencies may also speed or hinder
species displacement. For example, host plants are projected to move
much more slowly than most herbivorous insects, substantially slowing
displacement of the insects if they are unable to switch host plants
(Schweiger et al., 2012). Likewise, many habitats are structured by slow
moving plants, so habitat shifts are projected to lag behind climate
change (Hickler et al., 2012; Jones et al., 2012), which will in turn mediate
the movements of habitat specialists.
There are significant uncertainties in climate velocities, measured estimates
of dispersal and establishment rates, and model formulations. Climate
velocities are calculated using a variety of methods and spatial resolutions,
making direct comparisons difficult and leading to low confidence in
estimates of climate velocities in Figure 4-5b (limited evidence and
medium agreement). The lowest estimates of global average climate
velocity (Figure 4-5b, center axis) are about half the best estimate values
we show on the climate velocity axes (Loarie et al., 2009), while the
highest estimates are about four times higher (Burrows et al., 2011),
but high estimates may be artefacts of using very large spatial resolutions
(Dobrowski et al., 2013). In addition, the climate velocities used in Figure
4-5 are based on temperature alone, and recent analyses indicate that
including more climate factors increases climate velocity (Feeley and
Rehm, 2012; Dobrowski et al., 2013). Species displacement rates are
calculated based on a very wide range of methods including rates of
d
isplacement in the paleontological record, rates of current range shifts
due to climate warming, models of dispersal and establishment, maximum
observed dispersal distances and genetic analyses (e.g., Kinlan and
Gaines, 2003; Stevens, V.M. et al., 2010). There are often large differences
in estimates of dispersal rates across methods due to intrinsic uncertainties
in the methods and differences in the mechanisms included (Kinlan and
Gaines, 2003; Stevens, V.M. et al., 2010). For example, estimates of tree
displacement rates are frequently based on models or observations
that explicitly or implicitly include both dispersal of seeds and biotic
and abiotic factors controlling establishment of adult trees. Displacement
rates of trees are often more strongly limited by establishment than
dispersal (Higgins et al., 2003; Meier et al., 2012). It is reasonable to
expect that limits on establishment could also be important for other
species groups, but often only dispersal rates have been calculated,
leading to an overestimation of displacement rates. For trees there is
medium confidence in projections of their displacement rates due to
the large number of studies of past, current, and future displacement
rates (robust evidence and medium agreement). Less is known for other
broad species groups such as mammals, so there is only low confidence
in estimates of their displacement capacity. Estimates for other groups,
such as freshwater molluscs, are based on very little data, so estimates
of their dispersal capacity are poorly constrained.
Despite large uncertainties in displacement capacity and climate velocity,
the rates of displacement required to track the highest rates of climate
change (RCP8.5) are so high that many species will be unable to do so
(high confidence). Moderate rates of projected climate change (RCP4.5
and RCP6.0) would allow more species to track climate, but would still
exceed the capacity of many species to track climate (medium confidence).
The lowest rates of projected climate change (RCP2.6) would allow
most species to track climate toward the end of the century (high
confidence). This analysis highlights the importance of rates of climate
change as an important component of climate change impacts on
species and ecosystems. For example, differences in the magnitude of
climate change between scenarios are small at mid-21st century (WGI
AR5 Chapter 12), but the differences in rates of climate change are
large. At mid-century, it is projected that species would need to move
little at the lowest rates of climate change (RCP2.6), but will need to
move approximately 70 km per decade in flat areas in order to track
climate at the highest rates of climate change (RCP8.5).
Species that cannot move fast enough to keep pace with the rate of
climate change will lose favorable climate space and experience large
range contractions (Warren et al., 2013), whereas displacement that
keeps pace with climate change greatly increases the fraction of species
that can maintain or increase their range size (Menéndez et al., 2008;
Pateman et al., 2012). Mountains provide an extremely important
climate refuge for many species because the rate of displacement
required to track climate is low (Figure 4-5b; Colwell et al., 2008; Engler
et al., 2011; Gottfried et al., 2012; Pauli et al., 2012; but see Dullinger
et al., 2012). However, species that already occur near mountaintops
(or other boundaries) are among the most threatened by climate change
because they cannot move upwards (Ponniah and Hughes, 2004; Thuiller
et al., 2005; Raxworthy et al., 2008; Engler et al., 2011; Sauer et al.,
2011). The consequences of losing favorable climate space are not yet
well understood. The extent to which adaptive responses might allow
persistence in areas of unfavorable climates is discussed in Section

299
Terrestrial and Inland Water Systems Chapter 4
4
4
.4.1.2. In the absence of adaptation, losing favorable climate space is
projected to lead to reduced fitness, declining abundance, and local
extinction, with potentially large effects on biodiversity and ecosystem
services (see evidence of early signs of this for trees in Box 4-2).
4.3.2.5.3. Observed changes in abundance and local extinctions
Observations of range shifts imply changes in abundance, that is,
colonization at the “leading edge” and local extinction at the “trailing
edge” of ranges. Evidence that the attribution of these responses to
recent changes in climate can be made with high confidence for several
species groups is reviewed here (Section 4.3.2.5), in AR4, and by Cahill
et al. (2013). Changes in abundance, as measured by changes in the
population size of individual species or shifts in community structure
within existing range limits, have also occurred in response to recent
global warming (high confidence; Thaxter et al., 2010; Bertrand et al.,
2011; Naito and Cairns, 2011; Rubidge et al., 2011; Devictor et al., 2012;
Tingley et al., 2012; Vadadi-Fulop et al., 2012; Cahill et al., 2013; Ruiz-
Labourdette et al., 2013). Confident attribution to recent global warming
is hindered by confounding factors such as disease, land use change,
and invasive species (Cahill et al., 2013). New tentative conclusions
since AR4 are that climate-related changes in abundance and local
extinctions appear to be more strongly related to species interactions
than to physiological tolerance limits (low confidence; Cahill et al.,
2013) and that precipitation can be a stronger driver of abundance
change than temperature in many cases (Tian et al., 2011; Tingley et al.,
2012). This gives weight to concerns that biological interactions, which
are poorly known and modeled, may play a critical role in mediating
the impacts of future climate change on species abundance and local
extinctions (Dunn et al., 2009; Bellard et al., 2012; Hannah, 2012; Urban
et al., 2012; Vadadi-Fulop et al., 2012).
A few examples illustrate the types of change in abundance that are
being observed and the challenges in attributing these to recent global
warming. Some of the clearest examples of climate-related changes in
species populations come from high-latitude ecosystems where non-
climate drivers are of lesser importance. For example, both satellite data
and a large number of long-term observations indicate that shrub
abundance is generally increasing over broad areas of Arctic tundra,
which is coherent with predicted shifts in community structure due to
warming (Epstein et al., 2007; Goetz et al., 2011; Myers-Smith et al.,
2011). In the Antarctic, two native vascular plants, Antarctic pearlwort
(Colobanthus quitensis) and Antarctic hair grass (Deschampsia antarctica),
have become more prolific over recent decades, perhaps because they
benefit more from warming of soils than do mosses (Hill et al., 2011).
Penguin populations have declined in several areas of the Antarctic,
including a recent local extinction of an Emperor penguin (Aptenodytes
forsteri) population that has been attributed to regional changes in
climate (Trathan et al., 2011). The attribution of these declines to
changes in regional climate is well supported, but the link to global
warming is tenuous (Barbraud et al., 2011).
Mountains also provide good examples of changes in abundance that
can be linked to climate because very strong climate gradients are found
there. AR4 highlighted these responses, and the case for changes in
abundance, in particular plants, has become stronger since then. For
e
xample, Pauli et al. (2012) reported an increase in species richness from
plant communities of mountaintops in the European boreal and
temperate zones due to increasing temperatures and a decrease in
species richness on the Mediterranean mountain tops, probably due to a
decrease in the water availability in southern Europe. An increase in the
population size of warm-adapted species at high altitudes also appears
to be attributable to increasing temperatures (Gottfried et al., 2012).
However, these attributions are complicated by other anthropogenic
influences such as changes in grazing pressure, atmospheric N deposition,
and forest management practices (Gottfried et al., 2012). Altitudinal
gradients in local and global extinctions of amphibians also contributed
to the attribution of these extinctions to recent global warming, although
this attribution remains controversial (see Section 4.3.2.5.5).
4.3.2.5.4. Projected changes in abundance and local extinction
Ecological niche models do not predict population changes, but the
shifts in suitable climates can be used to infer areas where species
populations might decline or increase. These models project that local
extinction risk by the end of the 21st century due to climate change will
vary widely, ranging from almost no increase in local extinction risk
within the current range for some species or species groups to greatly
increased risk of local extinctions in more than 95% of the present-day
range for others (Settele et al., 2008; Bellard et al., 2012). Projected
local colonization rates are equally variable. There has been progress in
coupling species distribution models and species abundance models for
a wide range of organisms (Keith et al., 2008; Midgley et al., 2010;
Matthews et al., 2011; Schippers et al., 2011; Oliver et al., 2012a;
Renwick et al., 2012). These hybrid approaches predict extinction risk
directly, rather than by inference from changes in climate suitability
(Fordham et al., 2012). The main conclusions from these studies are that
changes in species abundance and local extinction risk as a result of
climate change can range from highly positive to highly negative, and
are determined by a combination of factors, including its environmental
niche, demographics, and life history traits, as well as interactions
among these factors (Aiello-Lammens et al., 2011; Clavero et al., 2011;
Conlisk et al., 2012; Fordham et al., 2012; Swab et al., 2012).
Changes in abundances will also be accompanied by changes in genetic
diversity (see also Section 4.4.1.2). At the intraspecific level, future climate
change is projected to induce losses of genetic diversity when it results
in range contraction (Balint et al., 2011; Pauls et al., 2013). In addition,
there is theoretical and observational evidence this loss of genetic
diversity will depend on rates of migration and range contraction
(Arenas et al., 2012). In these cases, reductions in genetic diversity may
then decrease the ability of species to adapt to further climate change
or other global changes. Climate change may also compound losses of
genetic diversity that are already occurring due to other global changes
such as the introduction of alien species or habitat fragmentation
(Winter et al., 2009; see also Section 4.2.4.6).
4.3.2.5.5. Observed global extinctions
Global species extinctions, many of them caused by human activities,
are now occurring at rates that approach or exceed the upper limits of

300
Chapter 4 Terrestrial and Inland Water Systems
4
o
bserved natural rates of extinction in the fossil record (Barnosky et al.,
2011). However, across all taxa there is only low confidence that rates
of species extinctions have increased over the last several decades
(birds: Szabo et al., 2012; but see Kiesecker, 2011, for amphibians). Most
extinctions over the last several centuries have been attributed to habitat
loss, overexploitation, pollution, or invasive species, and these are the
most important current drivers of extinctions (Millennium Ecosystem
Assessment, 2005b; Hofmann and Todgham, 2010; Cahill et al., 2013). Of
the more than 800 global extinctions documented by the International
Union for Conservation of Nature (IUCN), only 20 have been tenuously
linked to recent climate change (Cahill et al., 2013; see also Hoffmann
et al., 2010; Szabo et al., 2012). Molluscs, especially freshwater molluscs,
have by far the highest rate of documented extinctions of all species
groups (Barnosky et al., 2011). Mollusc extinctions are attributed
primarily to invasive species, habitat modification, and pollution;
changes in climate are rarely evoked as a driver (Lydeard et al., 2004;
Regnier et al., 2009; Chiba and Roy, 2011; but see a few cases in Kappes
and Haase, 2012; Cahill et al., 2013). Freshwater fish have the highest
documented extinction rates of all vertebrates, and again very few have
been attributed to changing climate, even tenuously (Burkhead, 2012;
Cahill et al., 2013). In contrast, changes in climate have been identified
as one of the key drivers of extinctions of amphibians (Pounds et al., 2006).
There have been more than 160 probable extinctions of amphibians
documented over the last 2 decades, many of them in Central America
(Pounds et al., 2006; Kiesecker, 2011). The most notable cases have been
the golden toad (Bufo periglenes) and Monteverde harlequin frog
(Atelopus varius) of Central America, which belong to a group of
amphibians with high rates of extinction previously ascribed to global
warming with “very high confidence” (Pounds et al., 2006; Fischlin et
al., 2007). This case has raised a number of important issues about
attribution because (1) the proximate causes of extinction of these and
other Central American frogs appear to be an extremely virulent invasive
fungal infection and land use change, with regional changes in climate
as a potential contributing factor, and (2) changes in regional climate
may have been related to natural climate fluctuations rather than
anthropogenic climate change (Sodhi et al., 2008; Lips et al., 2008;
Anchukaitis and Evans, 2010; Bustamante et al., 2010; Collins, 2010;
Vredenburg et al., 2010; Kiesecker, 2011; McKenzie and Peterson, 2012;
McMenamin and Hannah, 2012). Owing to low agreement among
studies there is only medium confidence in detection of extinctions and
attribution of Central American amphibian extinctions to climate
change. While this case highlights difficulties in attribution of extinctions
to recent global warming, it also points to a growing consensus that it
is the interaction of climate change with other global change pressures
that poses the greatest threat to species (Brook et al., 2008; Pereira et
al., 2010; Hof et al., 2011b). Overall, there is very low confidence that
observed species extinctions can be attributed to recent climate warming,
owing to the very low fraction of global extinctions that have been
ascribed to climate change and tenuous nature of most attributions.
4.3.2.5.6 Projected future species extinctions
Projections of future extinctions due to climate change have received
considerable attention since AR4. AR4 stated with medium confidence
“that approximately 20–30% of the plant and animal species assessed
to date are at increasing risk of extinction as global mean temperatures
e
xceed a warming of 2-3°C above preindustrial levels” (Fischlin et al.,
2007). All model-based analyses since AR4 broadly confirm this concern,
leading to high confidence that climate change will contribute to
increased extinction risk for terrestrial and freshwater species over the
coming century (Pereira et al., 2010; Sinervo et al., 2010; Pearson, 2011;
Warren et al., 2011, 2012; Bellard et al., 2012; Hannah, 2012; Ihlow et
al., 2012; Sekercioglu et al., 2012; Wearn et al., 2012; Foden et al., 2013).
Most studies indicate that extinction risk rises rapidly with increasing
levels of climate change, but some do not (Pereira et al., 2010). The
limited number of studies that have directly compared land use and
climate change drivers have concluded that projected land use change
will continue to be a more important driver of extinction risk throughout
the 21st century (Pereira et al., 2010). There is, however, broad agreement
that land use, and habitat fragmentation in particular, will pose serious
impediments to species adaptation to climate change as it is projected
to reduce the capacity of many species to track climate (see Section
4.3.2.5.3). These considerations lead to the assessment that future
species extinctions are a high risk because the consequences of climate
change are potentially severe, widespread, and irreversible, as extinctions
constitute the permanent loss of unique life forms.
There is, however, low agreement concerning the overall fraction of
species at risk, the taxa and places most at risk, and the time scale for
climate change-driven extinctions to occur. Part of this uncertainty arises
from differences in extinction risks within and between modeling studies:
this uncertainty has been evaluated in AR4 and subsequent syntheses
(Pereira et al., 2010; Warren et al., 2011; Bellard et al., 2012; Cameron,
2012). All studies project increased extinction risk by the end of the 21st
century due to climate change, but as indicated in AR4 the range of
estimates is large. Recent syntheses indicate that model-based estimates
of the fraction of species at substantially increased risk of extinction
due to 21st century climate change range from below 1% to above 50%
of species in the groups that have been studied (Pereira et al., 2010;
Bellard et al., 2012; Cameron, 2012; Foden et al., 2013). Differences in
modeling methods, species groups, and climate scenarios between
studies make comparisons between estimates difficult (Pereira et al.,
2010; Warren et al., 2011; Cameron, 2012).
Many papers published since AR4 argue that the uncertainty may be
even higher than indicated in syntheses of model projections, due to
limitations in the ability of current models to evaluate extinction risk (e.g.,
Kuussaari et al., 2009; Pereira et al., 2010; Dawson et al., 2011; McMahon
et al., 2011; Pearson, 2011; Araujo and Peterson, 2012; Bellard et al., 2012;
Fordham et al., 2012; Hannah, 2012; Kramer et al., 2012; Zurell et al.,
2012; Halley et al., 2013; Moritz and Agudo, 2013). Models frequently
do not account for genetic and phenotypic adaptive capacity, dispersal
capacity, population dynamics, the effects of habitat fragmentation and
loss, community interactions, micro-refugia, and the effects of rising CO
2
concentrations, all of which could play a major role in determining
species vulnerability to climate change, causing models to either over-
or underestimate risk. In addition, difficulties in model validation, large
variation in the climate sensitivity of species groups, and uncertainties
about time scales linking extinction risks to range reductions also lead
to large uncertainty in model-based estimates of extinction risk.
A variety of studies since AR4 illustrate how accounting for these factors
alters estimates of extinction risk. Accounting for biotic interactions

301
Terrestrial and Inland Water Systems Chapter 4
4
s
uch as pollination or predator-prey networks can increase modeled
extinction risks, at least for certain areas and species groups (Schweiger
et al., 2008; Urban et al., 2008; Hannah, 2012; Nakazawa and Doi,
2012), or can decrease extinction risk (Menéndez et al., 2008; Pateman
et al., 2012). Accounting for climatic variation at fine spatial scales may
increase (Randin et al., 2009; Gillingham et al., 2012; Suggitt et al., 2012;
Dobrowski et al., 2013; Franklin et al., 2013) or decrease (Trivedi et al.,
2008; Engler et al., 2011; Shimazaki et al., 2012) the persistence of small
populations under future climate change. Several recent studies indicate
that correlative species distribution models (the type of model most
frequently used for evaluating species extinction risk) tend to be much
more pessimistic concerning plant species range contractions and the
inferred extinction risks due to climate change when compared to
mechanistic models that explicitly account for the interactions between
climate change and protective effects of rising CO
2
concentrations on
plants (Morin and Thuiller, 2009; Kearney et al., 2010; Cheaib et al.,
2012). Models that account for population dynamics indicate that some
species populations, such as those of polar bears (Hunter et al., 2010),
will decline precipitously over the course of the next century due to
climate change, greatly increasing extinction risk, while others may not
(Keith et al., 2008). Phenotypic plasticity in one very well-studied
temperate bird population has been estimated to be sufficient to keep
extinction risk low even with projected warming exceeding 2–3°C
(Vedder et al., 2013), but this and other studies suggest that capacity for
adaptation is often substantially lower in species with long generation
times (see Section 4.4.1.2). There is evidence that interactions between
physiological tolerances and regional climate change will lead to large
taxonomic and spatial variation in extinction risk (Deutsch et al., 2008;
Sinervo et al., 2010). Even species whose populations are not projected
to decline rapidly over the next century can face a substantial “extinction
debt,” that is, will be in unfavorable climates that over a period of many
centuries are projected to lead to large reductions in population size and
increase the risk of extinction (Dullinger et al., 2012). Finally, evidence
from the paleontological record indicating very low extinction rates over
the last several hundred thousand years of substantial natural fluctuations
in climate—with a few notable exceptions such as large land animal
extinctions during the Holocene—has led to concern that forecasts of
very high extinction rates due entirely to climate change may be
overestimated (Botkin et al., 2007; Dawson et al., 2011; Hof et al.,
2011a; Willis and MacDonald, 2011; Moritz and Agudo, 2013). However,
as indicated in Section 4.2.3, no past climate changes are precise
analogs of future climate change in terms of speed, magnitude, and
spatial scale; nor did they occur alongside the habitat modification,
overexploitation, pollution, and invasive species that are characteristic
of the 21st century. Therefore the paleontological record cannot easily
be used to assess future extinction risk due to climate change.
4.3.3. Impacts on and Risks for Major Systems
This section covers impacts of climate change on broad categories of
terrestrial and freshwater ecosystems of the world. We have placed a
particular emphasis on those ecosystems that have high exposure to
climate change or that may be pushed past thresholds or “tipping
points” by climate change. Two geographical regions of particularly high
risk have been identified in recent studies: (1) tropics, due to the limited
capacity of species to adapt to moderate global warming and (2) high
n
orthern latitude systems, because temperature increases are projected
to be large. There has been a tendency to oppose these two points of
view, but there is a high risk in both types of systems, albeit for different
reasons (Corlett, 2011). Tropical species, which experienced low inter-
and intra-annual climate variability, have evolved within narrow thermal
limits, and are already near their upper thermal limits (ectotherms:
Deutsch et al., 2008; Huey et al., 2012; birds: Sekercioglu et al., 2012;
trees: Corlett, 2011). On this basis, tropical species and ecosystems are
predicted to be more sensitive to climate change than species and
ecosystems that have evolutionary histories of climatic variability (e.g.,
Arctic and boreal ecosystems; Beaumont et al., 2011). However, there
are physiological, evolutionary, and ecological arguments that tropical
species and ecosystem sensitivities to climate change are complex and
may not be particularly high compared to other systems (Gonzalez et
al., 2010; Corlett, 2011; Laurance et al., 2011; Gunderson and Leal, 2012;
Walters et al., 2012). High-latitude systems have the greatest projected
exposure to rising temperatures (WGI AR5 Chapter 12; Diffenbaugh and
Giorgi, 2012), which all else being equal would put them at higher risk.
The greatest degree of recent climate warming has occurred at high
northern latitudes (Burrows et al., 2011) and the strongest and clearest
signals of recent climate warming impacts on ecosystems come from
these regions. A comparison of modeled biome level vulnerability
indicated that temperate and high northern latitude systems are also
the most vulnerable in the future (Gonzalez et al., 2010).
Several potential tipping points (see Section 4.2.1) with regional and
global consequences have been identified (Scheffer, 2009); two are
elaborated in Boxes 4-3 (Amazon dieback) and 4-4 (tundra-boreal
regime shift). An assessment by the authors of this chapter of the top risks
in relation to climate change and terrestrial and freshwater ecosystems
is presented in Table 4-3.
4.3.3.1. Forests and Woodlands
Forests and woodlands are principal providers of timber, pulp, bioenergy,
water, food, medicines, and recreation opportunities and can play
prominent roles in cultural traditions. Forests are the habitat of a large
fraction of the Earth’s terrestrial plant and animal species, with the
highest concentrations and levels of endemism found in tropical regions
(Gibson et al., 2011). Climate change and forests interact strongly; air
temperature, solar radiation, rainfall, and atmospheric CO
2
concentrations
are major drivers of forest productivity and forest dynamics, and forests
help control climate through the large amounts of carbon they can remove
from the atmosphere or release, through absorption or reflection of
solar radiation (albedo), cooling through evapotranspiration, and the
production of cloud-forming aerosols (Arneth et al., 2010; Pan et al.,
2011; Pielke et al., 2011).
Combinations of ground-based observations, atmospheric carbon
budgets, and satellite measurements indicate with high confidence that
forests are currently a net sink for carbon at the global scale. It is
estimated that intact and regrowing forests currently contain 860 ± 70
PgC and sequestered 4.0 ± 0.7 PgC yr
–1
globally between 2000 and
2007 (WGI AR5 Chapter 6; Canadell et al., 2007; Pan et al., 2011; Le
Quéré et al., 2012). The carbon taken up by intact and regrowing forests
was counterbalanced by a release due to land use change of 2.8 ± 0.4
302
Chapter 4 Terrestrial and Inland Water Systems
4
PgC yr
–1
over this same period due mostly to tropical deforestation and
forest degradation associated with logging and fire, resulting in a net
carbon balance for global forests of 1.1±0.8 PgC yr
–1
.
The future of the interaction between climate and forests is unclear.
The carbon taken up by intact and regrowing forests appears to have
stabilized compared to the 1990s, after having increased in the 1970s
and 1980s (Canadell et al., 2007; Pan et al., 2011). There is medium
confidence that the terrestrial carbon sink is weakening. The drivers
behind the forest carbon sink vary greatly across regions. They include
forest regrowth and stimulation of carbon sequestration by climate
change, rising atmospheric CO
2
concentrations, and nitrogen deposition
Near term
(2030 – 2040)
Present
L
ong term
(
2080 – 2100)
2°C
4°C
Very
low
V
ery
high
Medium
N
ear term
(2030 – 2040)
Long term
(2080 – 2100)
T
able 4-3 | Key risks for terrestrial and freshwater ecosystems from climate change and the potential for reducing risk through mitigation and adaptation. Key risks are identified
based on assessment of the literature and expert judgments by chapter authors, with evaluation of evidence and agreement in supporting chapter sections. Each key risk is
characterized as very low to very high. Risk levels are presented in three time frames: the present, near term (here, assessed over 2030–2040), and longer term (here, assessed
over 2080–2100). For the near term era of committed climate change, projected levels of global mean temperature increase do not diverge substantially across emission
s
cenarios. For the longer term era of climate options, risk levels are presented for global mean temperature increase of 2°C and 4°C above pre-industrial levels. For each
t
imeframe, risk levels are estimated for a continuation of current adaptation and for a hypothetical highly adapted state. Relevant climate variables are indicated by icons. For a
g
iven key risk, change in risk level through time and across magnitudes of climate change is illustrated, but because the assessment considers potential impacts on different
physical, biological, and human systems, risk levels should not necessarily be used to evaluate relative risk across key risks, sectors, or regions.
Reduction in terrestrial carbon sink: Carbon stored in terrestrial ecosystems is vulnerable
t
o loss back into the atmosphere. Key mechanisms include an increase in fire frequency due to
climate change and the sensitivity of ecosystem respiration to rising temperatures.
(medium confidence)
[
4.2.4, 4.3.2, 4.3.3]
Adaptation prospects include managing
land use (including deforestation), fire,
a
nd other disturbances and non-climatic
stressors.
Present
2°C
4°C
Very
low
V
ery
high
Medium
Tree mortality and forest loss: Tree mortality has been observed to have increased in
many places and has been attributed in some cases to direct climate effects and indirect
effects due to pests and diseases. The dead trees increase the risk of forest fires.
(medium confidence)
[4.3.3.1, Box 4-2]
Adaption options include more effective
management of fire, pests, and
pathogens.
Present
2°C
4°C
Very
low
Very
high
Medium
Increased risk of species extinction: A large fraction of the species that have been assessed are
vulnerable to extinction as a result of climate change, often in interaction with other threats. Species
with an intrinsically low dispersal rate, especially when occupying flat landscapes where the projected
climate velocity is high, and species in isolated habitats such as mountain tops, islands, or small
protected areas are especially at risk. Cascading effects through organism interactions, and especially
those vulnerable to timing (phenological) changes, amplify the risk. (high confidence)
[
4.3.2.5, 4.3.3.3, 4.3.2.1, 4.4.2]
Adaptation options include reducing
habitat modification, habitat
fragmentation, pollution,
over-exploitation, and invasive species;
protected area expansion, assisted
dispersal, ex situ conservation.
Present
2°C
4°C
Very
low
Very
high
Medium
Invasion by non-native species: Disruptions of species interactions and the increase in
physiological stress as a result of being near the edge or outside of the historical climate niche
increases the vulnerability of ecosystems to invasion by non-native (alien) species, especially in the
presence of increased long-distance dispersal opportunities. In the extreme this can result in biome
shifts, with consequent changes in the spectrum of ecosystem services provided. (high confidence)
[4.2.4.6]
Climate is one driver among many.
Adaptation options are limited, largely
based on reducing other stresses and
measures to slow the unintended arrival of
aliens. Intensive direct intervention in
controlling emergent invasive species is an
option, but could be overwhelmed by the
rapidly rising number of cases.
Near term
(2030 – 2040)
Long term
(2080 – 2100)
Near term
(2030 – 2040)
Long term
(2080 – 2100)
Near term
(2030 – 2040)
Long term
(2080 – 2100)
Present
2°C
4°C
Very
l
ow
Very
h
igh
Medium
B
oreal tipping point: Arctic ecosystems are vulnerable to abrupt change related to the
thawing of permafrost and spread of shrubs in tundra and increase in pests and fires in
b
oreal forests. (medium confidence)
[
4.3.3.1.1, Box 4-4]
There are few adaptation options in the
Arctic.
Present
2°C
4°C
V
ery
low
Very
h
igh
M
edium
Amazon tipping point: Moist Amazon forests could change abruptly to less
carbon-dense drought and fire-adapted ecosystems. (low confidence)
[
4.3.3.1.3, Box 4-3]
Policy and market measures to reduce
deforestation and fire.
Near term
(2030 – 2040)
Long term
(2080 – 2100)
K
ey risk Adaptation issues & prospects
Climatic
drivers
R
isk & potential for
adaptation
T
imeframe
P
recipitation
C
limate-related drivers of impacts
W
arming
t
rend
E
xtreme
t
emperature
L
evel of risk & potential for adaptation
P
otential for additional adaptation
to reduce risk
R
isk level with
c
urrent adaptation
R
isk level with
h
igh adaptation
D
rying
t
rend

303
Terrestrial and Inland Water Systems Chapter 4
4
(
Pan et al., 2011; see also Sections 4.2.4.1, 4.2.4.2, 4.2.4.4). Most models
suggest that rising temperatures, drought, and fires will lead to forests
becoming a weaker sink or a net carbon source before the end of the
century (Sitch et al., 2008; Bowman et al., 2009). Fires play a dominant
role in driving forest dynamics in many parts of the world; forest
susceptibility to fire is projected to change little for the lowest emissions
scenario (RCP2.6), but substantially for the high emissions scenario
(RCP8.5; Figure 4-6). There is low agreement on whether climate change
will cause fires to become more or less frequent in individual locations
(Figure 4-6). Climate change-mediated disease and insect outbreaks
could exacerbate climate-driven increases in fire susceptibility (Kurz et
al., 2008). The greatest risks for large positive feedbacks from forests to
climate through changes in disturbance regimes arise from widespread
tree mortality and fire in tropical forests and low-latitude areas of boreal
forests, as well as northward expansion of boreal forests into Arctic
tundra (Lenton et al., 2008; Kriegler et al., 2009; Good et al., 2011b).
Recent evidence suggests (low confidence) that the stimulatory effects
of global warming and rising CO
2
concentrations on tree growth may
have already peaked in many regions (Charru et al., 2010; Silva et al.,
2010; Silva and Anand, 2013) and that warming and changes in
precipitation are increasing tree mortality in a wide range of forest
systems, acting via heat stress, drought stress, pest outbreaks, and a
wide range of other indirect impact mechanisms (Allen, C.D. et al., 2010;
Box 4-2). Detection of a coherent global signal is hindered by the lack
of long-term observations in many regions and attribution to climate
change is difficult because of the multiplicity of mechanisms mediating
mortality (Allen, C.D. et al., 2010).
Deforestation has slowed over the last decade (Meyfroidt and Lambin,
2011). This includes substantial reductions in tropical deforestation in
some regions, such as the Brazilian Amazon, where deforestation rates
declined rapidly after peaking in 2005 (Nepstad et al., 2009; INPE,
2013). Growing pressure for new crop (Section 4.4.4) and grazing land
will continue to drive tropical deforestation (medium confidence),
although recent policy experiments and market-based interventions in
land use demonstrate the potential to reduce deforestation (Meyfroidt
and Lambin, 2011; Westley et al., 2011; Nepstad et al., 2013).
4.3.3.1.1. Boreal forests
Most projections suggest a poleward expansion of forests into tundra
regions, accompanied by a general shift in composition toward more
temperate plant functional types (e.g., evergreen needleleaf being
replaced by deciduous broadleaf; or in colder regions, deciduous
needleleaf replaced by evergreen needleleaf (Lloyd et al., 2011; Pearson
et al., 2013). Projections of climate-driven changes in boreal forests over
the next few centuries remain uncertain on some issues, partly as a
result of different processes of change being considered in different
models. In particular, the inclusion or exclusion of fire and insects makes
a big difference, possibly making the boreal forest more susceptible to
a rapid, nonlinear, or abrupt decline in some regions (Bernhardt et al.,
2011; Mann et al., 2012; Scheffer et al., 2012; see WGI AR5 Chapter 12).
Recent observed change (Box 4-2) and dynamic vegetation modeling
(e.g., Sitch et al., 2008) suggest that regions of the boreal forest could
experience widespread forest dieback, although there is low confidence
o
wing to conflicting results (Sitch et al., 2008; Gonzalez et al, 2010) and
poor understanding of relevant mechanisms (WGI AR5 Section 12.5.5.6).
If such shifts were to occur, they would put the boreal carbon sink at
risk (Pan et al., 2011; Mann et al., 2012).
Whereas boreal forest productivity has been expected to increase as a
result of warming (Hari and Kulmata, 2008; Bronson et al., 2009; Zhao
and Running, 2010; Van Herk et al., 2011), and early analyses of satellite
observations confirmed this trend in the 1980s (medium confidence),
more recent and longer-term assessments indicate with high confidence
that many areas of boreal forest have instead experienced productivity
declines (high confidence; Goetz et al., 2007; Parent and Verbyla, 2010;
Beck, P.S.A. et al., 2011; de Jong et al., 2011). The best evidence to date
indicates that these “browning trends” are due to warming-induced
drought, specifically the greater drying power of air (vapor pressure
deficit; Williams et al., 2013), inducing photosynthetic down-regulation
of boreal tree species, particularly conifer species, most of which are not
adapted to the warmer conditions (Welp et al., 2007; Bonan, 2008; Van
Herk et al., 2011). Satellite evidence for warming-induced productivity
declines has been corroborated by tree ring studies (Barber et al., 2000;
Hogg et al., 2008; Beck, P.S.A. et al., 2011; Porter and Pisaric, 2011;
Griesbauer and Green, 2012) and long-term tree demography plots in
more continental and densely forested areas (Peng et al., 2011; Ma et
al., 2012). Conversely, productivity has increased at the boreal-tundra
ecotone, where more mesic (moist) conditions may be generating the
expected warming-induced positive growth response (Rupp et al., 2001;
McGuire et al., 2007; Goldblum and Rigg, 2010; Beck, P.S.A. et al., 2011).
The complexity of boreal forest response also involves tree age and size,
with younger trees and stands perhaps being more able to benefit from
warming where other factors are not limiting (Girardin et al., 2011, 2012).
Where they occur, warming and drying, coupled with productivity
declines, insect disturbance, and associated tree mortality, also favor
greater fire disturbance (high confidence). The boreal biome fire regime
has intensified regionally in recent decades, exemplified by increases in
the extent of area burned but also a longer fire season and more
episodic fires that burn with greater energy output or intensity (Girardin
and Mudelsee, 2008; Macias Fauria and Johnson, 2008; Kasischke et
al., 2010; Turetsky et al., 2011; Mann et al., 2012; Girardin et al., 2013a).
The latter is particularly important because more severe burning
consumes soil organic matter to greater depth, often to mineral soil,
providing conditions that favor recruitment of deciduous species that
in some regions of the North American boreal forest replace what was
previously evergreen conifer forest (Johnstone et al., 2010; Bernhardt
et al., 2011). Fire-mediated composition changes in post-fire succession
influence a host of ecosystem feedbacks to climate, including changes
in net ecosystem carbon balance (Bond-Lamberty et al., 2007; Goetz et
al., 2007; Welp et al., 2007; Euskirchen et al., 2009) as well as albedo
and energy balance (Randerson et al., 2006; Jin et al., 2012; O’Halloran
et al., 2012). The extent to which the net effect of these feedbacks will
exacerbate or mitigate additional warming is not well known over the
larger geographic domain of the boreal biome, except via modeling
studies that are relatively poorly constrained owing to sparse in situ
observations.
The vulnerability of the boreal biome to this cascading series of interacting
processes (Wolken et al., 2011), and their ultimate influence on climate
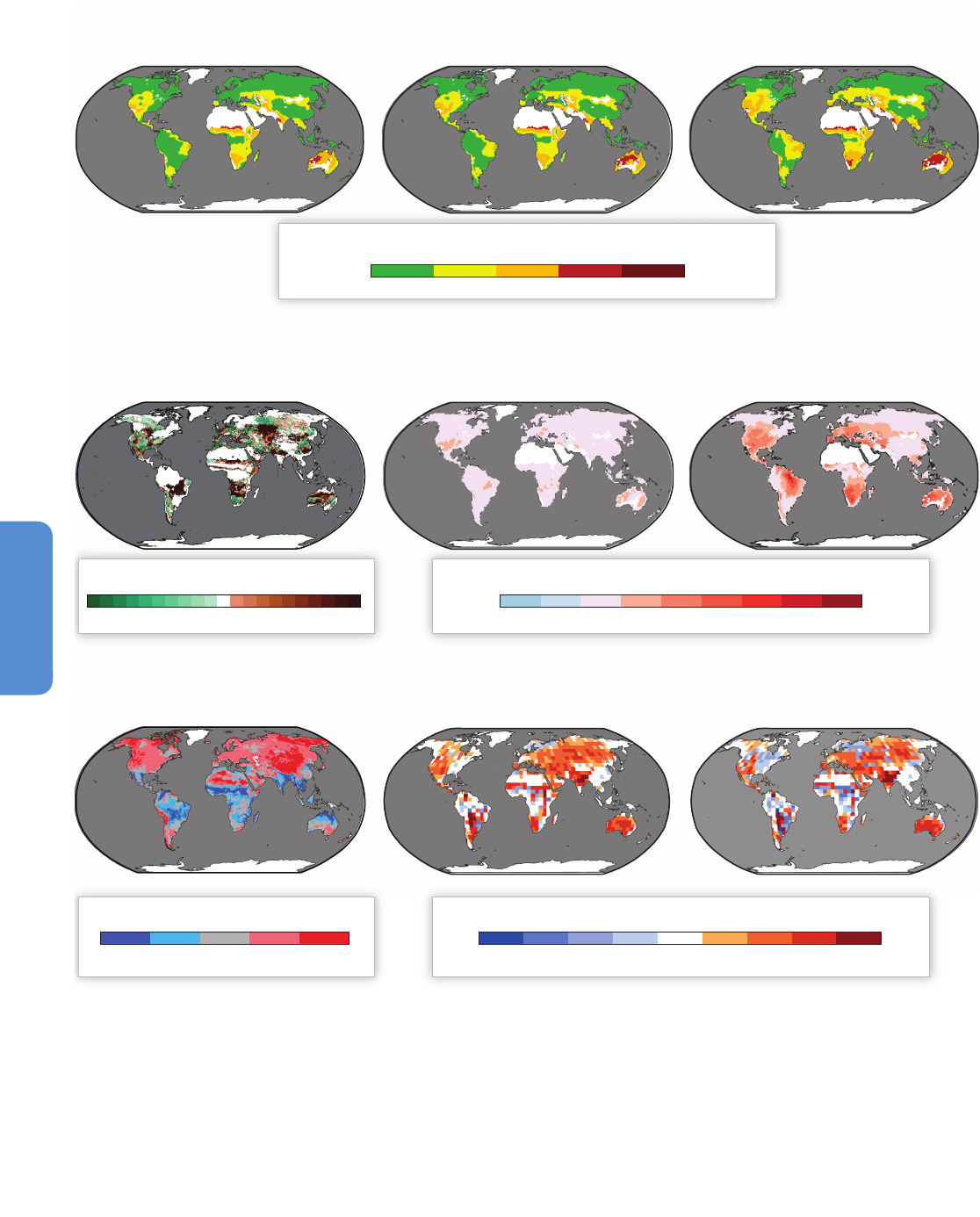
304
Chapter 4 Terrestrial and Inland Water Systems
4
Change in fire frequency (% per century)
Multi-model agreement
Multi-model agreement on change in
fire probability 1971–2000 to 2070–2099
–50 0 +50
5
–4
90%
–1000 –100
–10
–1
1
10 100 1000
Decrease Increase
67%
67% 90%
Low
agreement
–2
246
8 10
12
12 24 50
Forest Fire Danger Index (FFDI)
F
orest Fire Danger Index (FFDI)
Change in FFDI
Change in fire frequency
1951–2000 to 2051–2100
Change in Forest Fire Danger Index (FFDI) 1970–1999 to 2070–2099
Fire counts per year
Change in fire frequency between 2004 and 2100
1970–1999, HadGEM2-ES 2070–2099, RCP2.6, HadGEM2-ES
RCP2.6, HadGEM2-ES
A1B, three-model mean
(h) (i)
(
a)
(g)
(e)(d) (f)
(
b) (c)
A2
A2, GISSB1, GISS
2070–2099, RCP8.5, HadGEM2-ES
RCP8.5, HadGEM2-ES
Figure 4-6 | Projected changes in meteorological fire danger, fire probability, and fire frequency with different methods and climate models. (a) 30-year annual mean McArthur
Forest Fire Danger Index (FFDI) and change simulated with the Hadley Centre Global Environmental Model version 2 Earth System configuration (HadGEM2-ES) for 1970–1999,
with areas of no vegetation excluded (Betts et al., 2013). (b) As (a) for 2070–2099, Representative Concentration Pathway 2.6 (RCP2.6). (c) as (a) for 2070–2099, RCP8.5. (d)
Change in fire frequency by 2051–2100 relative to 1951–2000, SRES A1B, simulated with the MC1 vegetation model driven by three GCMs (Commonwealth Scientific and
Industrial Research Organisation (CSIRO)-Mk3.0, Met Office Hadley Centre Coupled Model version 3 (HadCM3), Model for Interdisciplinary Research On Climate (MIROC)
3.2medres; mean over three simulations; Gonzalez et al., 2010). (e) Difference between (b) and (a): change in FFDI by 2070–2099 relative to 1970–1999 in HadGEM2-ES,
RCP2.6. (f) Difference between (c) and (a): change in FFDI by 2070–2099 relative to 1970–1999 in HadGEM2-ES, RCP8.5. (g) Agreement on changes in fire probability by
2070–2099 relative to 1971–2000 (Moritz et al., 2012) simulated with a statistical model using climate projections from 16 Coupled Model Intercomparison Project Phase 3
(CMIP3) GCMs, Special Report on Emission Scenarios (SRES) A2. (h) Change in fire frequency by 2100 relative to 2004, SRES B1, simulated using climate and land cover
projections from the Goddard Institute of Space Studies General Circulation Model (GISS GCM) (AR4 version) and Integrated Model to Assess the Global Environment Integrated
Assessment Model (IMAGE IAM) (Pechony and Shindell, 2010). (i) As (h) for SRES A2. Changes in FFDI (a), (b), (c), (e), (f) and fire probability (g) arise entirely from changes in
meteorological quantities, whereas changes in fire frequency (d), (h), (i) depend on both meteorological quantities and vegetation.

305
Terrestrial and Inland Water Systems Chapter 4
4
f
eedbacks, differs between North America and northern Eurasia (high
confidence). The latter is dominated by deciduous conifer (larch) forest,
extending from western Russia across central to eastern Siberia—a
region more than twice the size of the North American boreal biome,
most of it underlain by permafrost. In terms of post-fire succession
analogous to the North American boreal biome, larch function more like
deciduous species than evergreen conifers, with greater density and
biomass gain in more severely burned areas, given adequate seed
survival through fire events or post-fire seed dispersal (Zyryanova, 2007;
Osawa et al., 2010; Alexander et al., 2012). Although the fire regime has
intensified in the last 100 years in Siberia, as well as in parts of North
America (Soja et al., 2007; Ali et al., 2012; Mann et al., 2012; Marlon et
al., 2013), the likelihood of regime shifts in larch forests is currently
unknown, partly because larch are self-replacing (albeit at different
densities) and partly because it is largely dependent on the fate of
permafrost across the region. In summary, an increase in tree mortality
is observed in many boreal forests, with the clearest indicators of this
in North America. However, tree health in boreal forests varies greatly
among regions, which coupled with insufficient temporal coverage
means that there is low confidence in the detection and attribution of
a clear temporal trend in tree mortality at the global scale (Figure 4-4).
The vulnerability of permafrost to thawing and degradation with climate
warming is critical not only for determining the rate of a boreal-tundra
biome shift and its associated net feedback to climate, but also for
predicting the degree to which the mobilization of very large carbon
stores frozen for centuries could provide additional warming (high
confidence; Schuur et al., 2008, 2009, 2013; Tarnocai et al., 2009;
Romanovsky et al., 2010; Schaefer et al., 2011; see WGI AR5 Chapters
6 and 12; see also Section 4.3.3.4). The extent and rate of permafrost
degradation varies with temperature gradients from warmer discontinuous
permafrost areas to colder, more continuous areas, but also with the
properties of the soil composition and biology (e.g., Mackelprang et al.,
2011). The degree of thermokarsting (melting of ice-rich soil) associated
with different substrates and associated topographic relief is variable
because boreal vegetation in later successional stages (evergreen conifers
in North America) insulates permafrost from air temperature increases;
soils with differing silt and gravel content tend to have different ice
content that, when melted, produces different degradation and
deformation rates; and because of other factors such as the reduction
of insulation provided by vegetation cover and soil organic layers due
to increased fire (Jorgenson et al., 2010; Grosse et al., 2011). This
variability and vulnerability is poorly represented in ESMs (McGuire et
al., 2012) and is thus the emphasis of research initiatives currently
underway. Carbon management strategies to keep permafrost intact,
for example, by removing forest cover to expose the land surface to
winter temperatures (Zimov et al., 2009), are impractical, not only
because of the vast spatial domain underlain by permafrost, but also
because of the broad societal and ecological impacts that would result.
4.3.3.1.2. Temperate forests
The largest areas of temperate forest are found in eastern North America,
Europe, and eastern Asia. The overall trend for forests in these regions
has until recently been an increase in growth rates of trees and in total
carbon stocks. This has been attributed to a combination of increasing
g
rowing season length, rising atmospheric CO
2
c
oncentrations, nitrogen
deposition, and forest management—specifically regrowth following
formerly more intensive harvesting regimes (Ciais et al., 2008). The
relative contribution of these factors has been the subject of substantial
and unresolved debate (Boisvenue and Running, 2006). Most temperate
forests are managed such that any change is and will be to a large
extent anthropogenic.
The world’s temperate forests act as an important carbon sink (high
confidence due to robust evidence and high agreement), absorbing 0.70
± 0.08 PgC yr
–1
from 1990 to 1999 and 0.80 ± 0.09 from 2000 to 2007
(Pan et al., 2011).This represents 34% of global carbon accumulation
in intact forests and 65% of the global net forest carbon sink (total sink
minus total emissions from land use).
Recent indications are that temperate forests and trees are beginning
to show signs of climate stress, including a reversal of tree growth
enhancement in some regions (North America: Silva et al., 2010; Silva
and Anand, 2013; Europe: Charru et al., 2010; Bontemps et al., 2011;
Kint et al., 2012); increasing tree mortality (Allen, C.D. et al., 2010; Box
4-2); and changes in fire regimes, insect outbreaks, and pathogen attacks
(Adams et al., 2012; Edburg et al., 2012). In northeastern France,
widespread recent declines in growth rates of European beech (Fagus
sylvatica L.) have been attributed to decreasing water availability
(Charru et al., 2010). These trends threaten the substantial role of
temperate forests as net carbon sinks, but it is still unclear to what
extent the observations are representative for temperate forests as a
whole. Several studies find that tree growth rates in temperate forests
passed their peak in the late 20th century and that the decline in tree
growth rates can be attributed to climatic factors, especially drought or
heat waves (Charru et al., 2010; Silva et al., 2010). Extreme climate
events have had a major impact on temperate forests over the last
decade (Ciais et al., 2005; Witte et al., 2011; Kasson and Livingston,
2012). Extensive forest fires occurred in Russia during the exceptionally
hot and dry summer of 2010 (Witte et al., 2011). The complex interactions
between climate and forest management in determining susceptibility
to extreme events make it difficult to unequivocally attribute these
events to recent climate warming (Allen, C.D. et al., 2010). There is
low confidence (limited evidence, medium agreement) that climate
change is threatening the temperate forest carbon sink directly or
indirectly.
At the biome level, there remains considerable uncertainty in the sign
and the magnitude of the carbon cycle response of temperate forests
to climate change. A comparison of Dynamic Global Vegetation
Models (DGVMs) showed that for identical end of 21st century climate
projections, temperate forests are variously projected to substantially
increase in total (biomass plus soil) carbon storage, especially through
gains in forest cover; or decrease due to reductions in total carbon
storage per hectare and loss of tree cover (Sitch et al., 2008). Projections
for eastern Asia are less variable: temperate forests remain carbon
sinks over the coming century, with carbon storage generally
peaking by mid-century and then declining (Sitch et al., 2008; Peng
et al., 2009; Ni, 2011). However, regional vegetation models for China
predict a substantial northward shift of temperate forest (Weng and
Zhou, 2006; Ni, 2011). There is little indication from either models or
observations that the responses of temperate forests to climate change
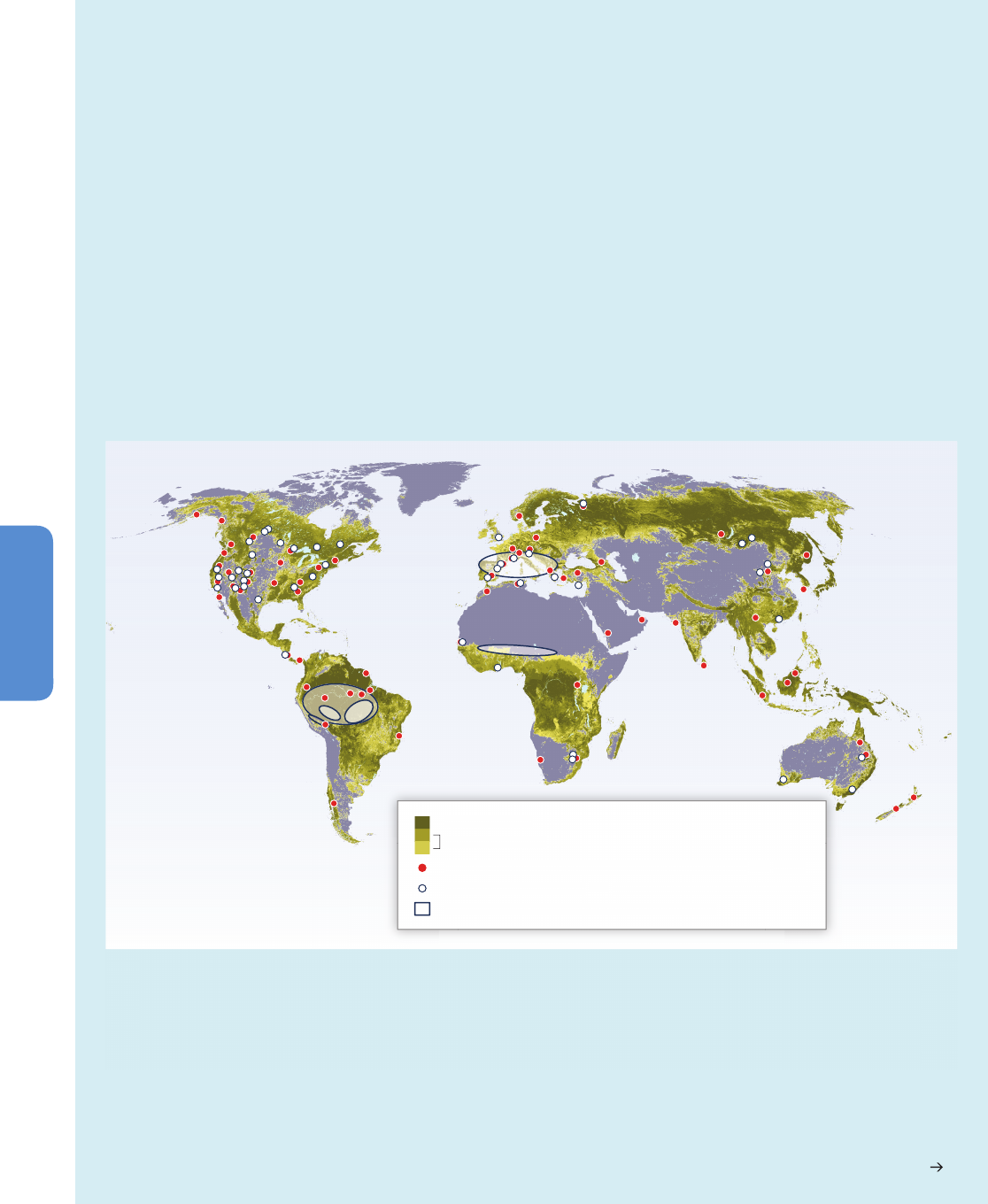
306
Chapter 4 Terrestrial and Inland Water Systems
4
Box 4-2 | Tree Mortality and Climate Change
Extensive tree mortality and widespread forest dieback (high mortality rates at a regional scale) linked to drought and temperature
stress have been documented recently on all vegetated continents (Allen, C.D. et al., 2010; Figure 4-7). However, appropriate field
data sets are currently lacking for many regions (Anderegg et al., 2013a), leading to low confidence in our ability to detect a global
trend. Nevertheless, long-term increasing tree mortality rates associated with temperature increases and drought have been
documented in boreal and temperate forests in western North America (van Mantgem et al., 2009; Peng et al., 2011). Increased levels
of tree mortality following drought episodes have also been detected in multiple tropical forests (Kraft et al., 2010; Phillips et al.,
2010) and Europe (Carnicer et al., 2011). Episodes of widespread dieback (high mortality rates at a regional scale) have been
observed in multiple vegetation types, particularly in western North America, Australia, and southern Europe (Raffa et al., 2008;
Carnicer et al., 2011; Anderegg et al., 2013a). Some widespread dieback events have occurred concomitant with infestation outbreaks
(Hogg et al., 2008; Raffa et al., 2008; Michaelian et al., 2011), where insect populations are also directly influenced by climate, such
as population release by warmer winter temperatures (Bentz et al., 2010). Although strong attribution of extensive tree mortality to
recent warming has been made in a few studies, the paucity of long-term studies of the mechanisms driving mortality means that
there is low confidence that this attribution can be made at the global scale.
Localities compiled through 2009 (summarized and listed in Allen et al., 2010)
Global forest cover
Other wooded regions
Examples not included in Allen et al., 2010, largely from post-2009 publications
Broad areas described by particular post-2009 publications
Localities c
omp
iled thro
ugh
2009
(su
mmarized and listed in Alle
n
et al
.
, 2010)
Global forest cove
r
Other wooded re
gio
ns
Examples not included in Allen
et al
.
, 2010, l
arg
ely
from
pos
t-2009
pu
blications
Broad areas described by
pa
rticular
pos
t-2009
pu
blication
s
Figure 4-7 | Locations of substantial drought- and heat-induced tree mortality around the globe since 1970 (global forest cover and other wooded regions based on
FAO, 2005). Studies compiled through 2009 (red dots) are summarized and listed in Allen, C.D. et al. (2010). Localities and measurement networks not included in Allen,
C.D. et al. (2010), which are largely from post-2009 publications, have been added to this map (white dots and shapes). New locality references by region: Africa: Mehl
et al., 2010; van der Linde et al., 2011; Fauset et al., 2012; Gonzalez et al., 2012; Kherchouche et al., 2012; Asia: Dulamsuren et al., 2009; Kharuk et al., 2013; Liu et al.,
2013; Zhou et al., 2013; Australasia: Brouwers et al., 2012; Fensham et al., 2012; Keith et al., 2012; Matusick et al., 2012; Brouwers et al., 2013; Matusick et al., 2013;
Europe: Innes, 1992; Peterken and Mountford, 1996; Linares et al., 2009; Galiano et al., 2010; Vennetier and Ripert, 2010; Aakala et al., 2011; Carnicer et al., 2011;
Linares et al., 2011; Sarris et al., 2011; Marini et al., 2012; Cailleret et al., 2013; Vilà-Cabrera et al., 2013; North America: Fahey, 1998; Minnich, 2007; Klos et al., 2009;
Ganey and Vojta, 2011; Michaelian et al., 2011; Peng et al., 2011; DeRose and Long, 2012; Fellows and Goulden, 2012; Kaiser et al., 2012; Millar et al., 2012; Garrity et
al., 2013; Kukowski et al., 2013; Williams et al., 2013; Worrall et al., 2013; South America: Enquist and Enquist, 2011; Lewis et al., 2011; Saatchi et al., 2013.
Continued next page

307
Terrestrial and Inland Water Systems Chapter 4
4
are characterized by tipping points (Bonan, 2008). There is low
confidence (medium evidence, low agreement) on long-term, climate-
driven changes in temperate forest biomass and geographical range
shifts.
At the species level, models predict that the potential climatic space for
most tree species will shift poleward and to higher altitude in response
to climate change (Dale et al., 2010; Ogawa-Onishi et al., 2010; Hickler
et al., 2012). Associated long-term projected range shifts generally vary
from several kilometers to several tens of kilometers per decade, most
probably faster than natural migration (e.g., Chmura et al., 2011; see
also Section 4.3.2.5). Therefore, assisted migration has been suggested
as an adaptation measure (see Section 4.4.2.4). Such shifts would alter
biodiversity and ecosystem services from temperate forests (e.g., Dale
et al., 2010). Multi-model comparisons for temperate forests, however,
illustrate that there are differences in species response and that models
differ greatly in the severity of projected climate change impacts on
species ranges (Morin and Thuiller, 2009; Kearney et al., 2010; Kramer
et al., 2010; Cheaib et al., 2012). Tree growth models project increased
tree growth at the poleward and high altitudinal range limits over most
of the 21st century in China (Ni, 2011). New approaches to modeling
tree responses, based on the sensitivity of key life-history stages, suggest
that climate change impacts on reproduction could be a major limitation
on temperate tree distributions (Morin et al., 2007). Comparisons with
paleoecological data have helped improve confidence in the ability of
models to project future changes in species ranges (Pearman et al.,
2008; Allen, J.R.M. et al., 2010; Garreta et al., 2010). Model projections
are qualitatively coherent with observations that temperate forest
species are moving up in altitude, probably due to climate warming at
the end of the 20th century (Lenoir et al., 2008). There is medium
confidence (medium evidence, medium agreement) that temperate tree
species are migrating poleward and to higher altitudes.
4.3.3.1.3. Tropical forests
Climate change effects on tropical forests interact with the direct
influences of humans and are understood largely through field studies
of the responses of forests to extreme weather events and through
models that are able to simulate a growing number of ecological and
atmospheric processes (Malhi et al., 2008; Davidson et al., 2012).
A key uncertainty in our understanding of future impacts of climate
change on tropical forests is the strength of direct CO
2
effects on
photosynthesis and transpiration (see Section 4.3.2.4). These responses
will play an important role in determining tropical forest trends as
temperatures and atmospheric CO
2
concentrations rise. There is a
physiological basis for arguing that photosynthesis will increase
sufficiently to offset the inhibitory effects of higher temperatures on
forest productivity (Lloyd and Farquhar, 2008), although heightened
photosynthesis does not necessarily translate into an increase in overall
forest biomass (Körner and Basler, 2010). DGVMs and the current
generation of ESMs, including those used within CMIP5 (e.g., Jones et
al., 2011; Powell et al., 2013), generally use formulations for CO
2
effects
on photosynthesis and transpiration based on laboratory-scale work
(Jarvis, 1976; Farquhar et al., 1980; Ball et al., 1987; Stewart, 1988;
Collatz et al., 1992; Leuning, 1995; Haxeltine and Prentice, 1996; Cox
et al., 1998) that predates larger ecosystem-scale studies, although
some models have been calibrated on the basis of more recent data
(Jones et al., 2011).
A second important source of uncertainty is the rate of future CO
2
rise and
climate change (Betts et al., 2012). Modeled simulations of future climate
in tropical forest regions indicate with high confidence (robust evidence,
high agreement) that temperature will increase. Future precipitation
change, in contrast, is highly uncertain and varies considerably between
Box 4-2 (continued)
Forest dieback has influenced the species composition, structure and age demographics, and successional trajectories in affected
forests, and in some cases led to decreased plant species diversity and increased risk of invasion (Kane et al., 2011; Anderegg et al.,
2012). Widespread tree mortality also has multiple effects on biosphere-atmosphere interactions and could play an important role in
future carbon-cycle feedbacks through complex effects on forest biophysical properties and biogeochemical cycles (Breshears et al.,
2005; Kurz et al., 2008; Anderson et al., 2011).
Projections of tree mortality due to climate stress and potential thresholds of widespread forest loss are currently highly uncertain
(McDowell et al., 2011). Most current vegetation models have little-to-no mechanistic representation of tree mortality (Fisher et al.,
2010; McDowell et al., 2011). Nonetheless, a global analysis of tree hydraulic safety margins found that 70% of surveyed tree species
operate close to their limits of water stress tolerance (Choat et al., 2012), indicating that vulnerability to drought and temperature
stress will not be limited to arid and semiarid forests. Furthermore, time scales of tree and plant community recovery following
drought are largely unknown, but preliminary evidence from several forests indicates that full recovery times may be longer than
drought return intervals, leading to “compounding” effects of multiple droughts (Mueller et al., 2005; Anderegg et al., 2013b; Saatchi
et al., 2013). Projected increases in temperature are also expected to facilitate expansion of insect pest outbreaks poleward and in
altitude, which may also cause or contribute to tree mortality (Bentz et al., 2010).

308
Chapter 4 Terrestrial and Inland Water Systems
4
c
limate models (WGI AR5 Annex 1: Atlas of Global and Regional Climate
Projections), although there is medium confidence (medium evidence,
medium agreement) that some tropical regions, such as the eastern
Amazon Basin, will experience lower precipitation and more severe
drought (Malhi et al., 2009a; Shiogama et al., 2011). The range of
possible shifts in the moist tropical forest envelope is large, sensitive to
the responsiveness of water use efficiency (WUE) to rising concentrations
of atmospheric CO
2
, and varies depending on the climate and vegetation
model that is used (Scholze et al., 2006; Sitch et al., 2008; Zelazowski
et al., 2011). Recent model studies (Malhi et al., 2009a; Cox et al., 2013;
Huntingford et al., 2013) indicate that the future geographical range
of moist tropical forests as determined by its shifting climatological
envelope is less likely to undergo major retractions or expansions by
2100 than was suggested in AR4. Since AR4, there is new evidence of
more frequent severe drought episodes in the Amazon region that are
associated with sea surface temperature increases in the tropical North
Atlantic (medium confidence; Marengo et al., 2011). There is low
confidence, however, that these droughts or the observed sea surface
temperatures can be attributed to climate change.
Networks of long-term forest plots reveal that lianas and fast-growing
tree species are increasing, as is forest biomass (Phillips et al., 2002,
2005; Lewis et al., 2009a,b, 2011). Faster tree growth is consistent with
increasing WUE associated with the rising concentration of CO
2
, but
also with changes in solar radiation and the ratio of diffuse to direct
radiation (Lewis et al., 2009a; Mercado et al., 2009; Brando et al., 2010;
see also Section 4.2.4.5). There is low confidence (limited evidence,
medium agreement) that the composition and biomass of Amazon and
African forests are changing through the rise in atmospheric CO
2
. The
potential suppression of photosynthesis and tree growth in tropical
forests through rising air temperatures is supported by physiological
and eddy covariance studies (Doughty and Goulden, 2008; Lloyd and
Farquhar, 2008; Wood et al., 2012), but is not yet observed as changes
in forest biomass (except Clark et al., 2003).
Since AR4, there is new experimental and observational evidence of
ecological thresholds of drought and fire in moist tropical forests that
points to an important indirect role of climate change in driving large-
scale changes in these ecosystems, and to the importance of extreme
drought events (see Box 4-3). Forest tree mortality increased abruptly
above a critical level of soil moisture depletion in two rainfall exclusion
experiments (Nepstad et al., 2007; Fisher et al., 2008) and above a critical
level of weather-related fire intensity in a prescribed burn experiment
(Brando et al., 2012). These experimental results were corroborated by
observations of increased tree mortality during the severe 2005 drought
in the Amazon (Phillips et al., 2009) and extensive forest fire (Alencar
et al., 2006, 2011; Aragão et al., 2008; Box 4-3). There is high confidence
(medium evidence, high agreement) that moist tropical forests have
many tree species that are vulnerable to drought- and fire-induced
mortality during extreme dry periods.
There is also a growing body of evidence that severe weather events
interact with land use to influence moist tropical forest fire regimes.
Many moist tropical forests are not susceptible to fire during typical
rainfall years because of high moisture content of fine fuels (Cochrane,
2003). Selective logging, drought, and fire itself can reduce this fire
resistance by killing trees, thinning the canopy, and allowing greater
h
eating of the forest interior (Uhl and Kauffman, 1990; Curran et al.,
2004; Ray et al., 2005; Box 4-3). Land use also often increases the
ignition sources in tropical landscapes (Silvestrini et al., 2011). These
relationships are not yet represented fully in coupled climate-vegetation
models. There is high confidence (robust evidence, high agreement) that
forest fire frequency and severity is increasing through the interaction
between severe droughts and land use. There is medium confidence
(medium evidence, high agreement) that tree mortality in the Amazon
region is increasing through severe drought and increased forest fire
occurrence and low confidence that this can be attributed to warming
(Figure 4-4).
Dry tropical forests are defined by strong seasonality in rainfall
distribution (Mooney et al., 1995) and have been reduced to an estimated
1 million km
2
globally through human activities (Miles et al., 2006). Half
of the world’s remaining dry tropical forests are located in South America.
Using five climate model simulations for the 2040–2069 period under
the IS92a “business-as-usual scenario,” Miles et al. (2006) found that
approximately one-third of the remaining area of tropical dry forests in
the Americas will be exposed to higher temperatures and lower rainfall
through climate change. Climate change, deforestation, fragmentation,
fire, or human pressure place virtually all (97%) of the remaining
tropical dry forests at risk of replacement or degradation (Miles et al.,
2006). In a regional study a dynamic vegetation model (Integrated
Biosphere Simulator (IBIS)) under A2 and B2 scenarios projected by a
global climate model (Hadley Centre Regional Model 3 (HadRM3))
found that most of the dry forests of India would be outside of their
climate envelopes later in this century (Chaturvedi et al., 2011). There
is low confidence in our understanding of climate change effects on dry
forests globally.
4.3.3.2. Dryland Ecosystems:
Savannas, Shrublands, Grasslands, and Deserts
The following sections treat a wide range of terrestrial ecosystems
covering a large part of the land surface, whose common features are
that they typically exhibit strong water stress for several months each
year and grass-like plants and herbs are a major part of their vegetation
cover. Thus the principal land use often involves grazing by domestic
livestock or wild herbivores.
4.3.3.2.1. Savannas
Savannas are mixtures of coexisting trees and grasses, covering about
a quarter of the global land surface, including tropical and temperate
forms. Savannas are characterized by annual to decadal fires (Archibald
et al., 2009) of relatively low intensity, which are an important factor
in maintaining the tree-grass proportions (Beerling and Osborne, 2006),
but also constitute a major and climate-sensitive global source of fire-
related emissions from land to atmosphere (Schultz et al., 2008; van
der Werf et al., 2010). The geographical distribution of savannas is
determined by temperature, the seasonal availability of water, fire, and
soil conditions (Ellery et al., 1991; Walker and Langridge, 1997; Staver
et al., 2011) and is therefore inferred to be susceptible to climate
change. In parts of Central Africa, forests have been observed to be
309
Terrestrial and Inland Water Systems Chapter 4
4
Box 4-3 | A Possible Amazon Basin Tipping Point
Since AR4, our understanding of the potential of a large-scale, climate-driven, self-reinforcing transition of Amazon forests to a dry
stable state (known as the Amazon “forest dieback”) has improved. Modeling studies indicate that the likelihood of a climate-driven
forest dieback by 2100 is lower than previously thought (Malhi et al., 2009b; Cox et al., 2013; Good et al., 2013; Huntingford et al.,
2013), although lower rainfall and more severe drought is expected in the eastern Amazon (Malhi et al., 2009a). There is now
medium confidence (medium evidence, medium agreement) that climate change alone (i.e., through changes in the climate envelope,
without invoking fire and land use) will not drive large-scale forest loss by 2100 although shifts to drier forest types are predicted in
the eastern Amazon (Mahli et al., 2009a). Meteorological fire danger is projected to increase in some models (Golding and Betts,
2008; Betts et al., 2013; Figure 4-6). Field studies and regional observations have provided new evidence of critical ecological
thresholds and positive feedbacks between climate change and land use activities that could drive a fire-mediated, self-reinforcing
dieback during the next few decades (Figure 4-8). There is now medium confidence (medium evidence, high agreement) that severe
drought episodes, land use, and fire interact synergistically to drive the transition of mature Amazon forests to low-biomass, low-
statured fire-adapted woody vegetation.
Amazon
River
Grass
invasion
Forest fires
Severe
drought
Global warming
Rising atmospheric CO
2
Logging and
clearing
Tree
death
Andes Mountains
Figure 4-8 | The forests of the Amazon Basin are being altered through severe droughts, land use (deforestation, logging), and increased frequencies of forest fire.
Some of these processes are self-reinforcing through positive feedbacks, and create the potential for a large-scale tipping point. For example, forest fire kills trees,
increasing the likelihood of subsequent burning. This effect is magnified when tree death allows forests to be invaded by flammable grasses. Deforestation provides
ignition sources to flammable forests, contributing to this dieback. Climate change contributes to this tipping point by increasing drought severity, reducing rainfall and
raising air temperatures, particularly in the eastern Amazon Basin (medium confidence; medium evidence, medium agreement).
Continued next page

310
Chapter 4 Terrestrial and Inland Water Systems
4
moving into adjacent savannas and grasslands (Mitchard et al., 2009),
possibly due to depopulation and changes in the fire regime. In northern
Australia, forest is expanding into former savanna areas (Brook and
Bowman, 2006; Bowman et al., 2011; Tng et al., 2012). It has been
projected that drying and greater seasonality, acting in conjunction with
increased fire, could lead to former forested areas becoming savannas
in parts of the Amazon basin (Malhi et al., 2009b; Box 4-3). In many
places around the world the savanna boundary is moving into former
grasslands on elevation gradients; in other words, into areas inferred
to be formerly too cool for trees (Breshears, 2006).
The proportion of trees and grasses in savannas is considered unstable
under some conditions (De Michele et al., 2011; Staver et al., 2011). The
differential effects of climate change, rising CO
2
, fire, and herbivory on
trees and grasses have the potential to alter the tree cover in savannas,
possibly abruptly. There is evidence from many parts of the world that
the tree cover and biomass in savannas has increased over the past
century and in some places, on all continents, continues to do so
(robust evidence, high agreement; Moleele et al., 2002; Angassa and
Oba, 2008; Cabral et al., 2009; Wigley et al., 2009; Witt et al., 2009; Lunt
et al., 2010; Rohde and Hoffman, 2012). The general consequences are
more carbon stored per unit land area in form of tree biomass and soil
organic matter (Hughes et al., 2006; Liao et al., 2006; Knapp et al., 2007;
Throop and Archer, 2008; Boutton et al., 2009), changes in hydrology
(Muñoz-Robles et al., 2011), and reduced grazing potential (Scholes and
Archer, 1997). Increasing tree cover in savannas has been attributed to
changes in land management (Joubert et al., 2008; Van Auken, 2009),
rising CO
2
(Bond and Midgley, 2012; Buitenwerf et al., 2012), climate
variability and change (Eamus and Palmer, 2007; Fensham et al., 2009),
or several of these factors acting in combination (Ward, 2005). As yet,
there are no studies that definitively attribute the relative importance
of the climate- and non-climate-related causes of woody plant biomass
increase in savannas (and the invasion of trees into former grasslands),
but there is medium agreement and robust evidence that climate
change and rising CO
2
are contributing factors in many cases. The
increased growth rate of C
3
photosynthetic system trees relative to C
4
Box 4-3 (continued)
Most primary forests of the Amazon Basin have damp fine fuel layers and low susceptibility to fire, even during annual dry seasons
(Uhl and Kauffman, 1990; Ray et al., 2005). Forest susceptibility to fire increases through canopy thinning and greater sunlight
penetration caused by tree mortality associated with selective logging (Uhl and Kauffman, 1990; Ray et al., 2005; Barlow and Peres,
2008), previous forest fire (Balch et al., 2008; Brando et al., 2012), severe drought (Alencar et al., 2006), or drought-induced tree
mortality (Nepstad et al., 2007; da Costa et al., 2010). The impact of fire on tree mortality is also weather dependent. Under very dry,
hot conditions, fire-related tree mortality can increase sharply (Brando et al., 2012). Under some circumstances, tree damage is
sufficient to allow light-demanding, flammable grasses to establish in the forest understory, increasing forest susceptibility to further
burning (Veldman and Putz, 2011). There is high confidence (robust evidence, high agreement) that logging, severe drought, and
previous fire increase Amazon forest susceptibility to burning.
Landscape level processes further increase the likelihood of forest fire. Fire ignition sources are more common in agricultural and
grazing lands than in forested landscapes (Silvestrini et al., 2011) (high confidence: robust evidence, high agreement), and forest
conversion to grazing and crop lands can inhibit regional rainfall through changes in albedo and evapotranspiration (Costa et al.,
2007; Butt et al., 2011; Knox et al., 2011) (low confidence: medium evidence, low agreement) or through smoke, which can inhibit
rainfall under some circumstances (Andreae et al., 2004) (medium confidence: medium evidence, medium agreement). Apart from
these landscape processes, climate change could increase the incidence of severe drought episodes (Mahli et al. 2009b; Shiogama et
al., 2011).
If recent patterns of deforestation (through 2005), logging, severe drought, and forest fire continue into the future, more than half of
the region’s forests will be cleared, logged, burned, or exposed to drought by 2030, even without invoking positive feedbacks with
regional climate, releasing 20 ± 10 PgC to the atmosphere (Nepstad et al., 2008) (low confidence: low evidence, medium agreement)
(Figure 4-8). The likelihood of a tipping point being reached may decline if extreme droughts (such as 1998, 2005, and 2010)
(Marengo et al., 2011) become less frequent, if land management fires are suppressed, if forest fires are extinguished on a large scale
(Soares-Filho et al., 2012), if deforestation declines, or if cleared lands are reforested (Nepstad et al., 2008). The 77% decline in
deforestation in the Brazilian Amazon with 80% of the region’s forest still standing (INPE, 2013) demonstrates that policy-led
avoidance of a fire-mediated tipping point is plausible.

311
Terrestrial and Inland Water Systems Chapter 4
4
g
rasses under rising CO
2
c
ould relieve the demographic bottleneck that
keeps trees trapped within the flame zone of the grasses, a hypothesis
supported by elevated CO
2
experiments with savanna saplings (Kgope
et al., 2010).
A model of grasslands, savannas, and forests suggests that rising CO
2
does increase the likelihood of abrupt shifts to woodier states, but the
transition will take place at different CO
2
concentrations in different
environments (Higgins and Scheiter, 2012). On the other hand,
observation of contrasts in the degree of savanna thickening between
land parcels with the same CO
2
exposure but different land use histories,
topographic position, or soil depth (Wiegand et al., 2005; Wu and Archer,
2005) imply that land management, water balance, and microclimate
are also important. Tree cover in savannas is rainfall-constrained
(Sankaran et al., 2005), suggesting that future increases in rainfall
projected for most but not all savanna areas (WGI AR5 Annex I: Atlas
of Global and Regional Climate Projections) could lead to increased tree
biomass.
4.3.3.2.2. Grasslands and shrublands
Rangelands (partly overlapping with savannas) cover approximately
30% of the Earth’s ice-free land surface and hold an equivalent amount
of the world’s terrestrial carbon (Booker et al., 2013). Much evidence
from around the world shows that dry grasslands and shrublands are
highly responsive in terms of primary production, species composition,
and carbon balance to changes in water balance (precipitation and
evaporative demand) within the range of projected climate changes
(high confidence) (e.g., Sala et al., 1988; Snyman and Fouché, 1993; Fay
et al., 2003; Peñuelas et al., 2004, 2007; Prieto et al., 2009; Peters et al.,
2010; Martí-Roura et al., 2011; Booker et al., 2013; Wu and Chen, 2013).
Rainfall amount and timing have large effects on a wide range of
biological processes in grasslands and shrublands, including seed
germination, seedling establishment, plant growth, flowering time, root
mass, community composition, population and community dynamics
production, decomposition and respiration, microbial processes and
carbon, plant, and soil nutrient contents (e.g., Fay et al., 2003; Peñuelas
et al., 2004, 2007; Beier et al., 2008; Sardans et al., 2008a,b; Sowerby
et al., 2008; Liu et al., 2009; Miranda et al., 2009; Albert et al., 2011,
2012; Selsted et al., 2012; Walter et al., 2012).
Precipitation changes were as important for mountain flora in Europe
as temperature changes, and the greatest composition changes will
probably occur when decreased precipitation accompany warming
(Engler et al., 2011). Responses of shrublands to drought may be driven
partly by changes in the soil microbial community (Jensen et al., 2003)
or changes in soil fauna (Maraldo et al., 2008). An increase in drought
frequency, without an increase in drought severity, leads to loss of soil
carbon in moist, carbon-rich moorlands, due to changes in soil structure
or soil microbial community leading to increased hydrophobicity and
soil respiration (Sowerby et al., 2008, 2010). Simulated increased spring
temperature and decreased summer precipitation had a general negative
effect on plant survival and plant growth, irrespective of the macroclimatic
niche characteristics of the species. Against expectation, species with
ranges extending into drier regions did not generally perform better
under drier conditions (Bütof et al., 2012).
C
hanging climate and land use have resulted in increased aridity and
a higher frequency of droughts in drylands around the world, with
increasing dominance of abiotic controls of land degradation (in contrast
to direct human- or herbivore-driven degradation) and changes in
hydrology and the erosion of soil by wind (Ravi et al., 2010). In mixed shrub
grasslands, the influence of drought periods could produce transient
pulses of carbon that are much larger than the pulses produced by fire
(Martí-Roura et al., 2011). Most studies of changes in arid systems between
grasslands and shrublands have focused on plant-soil feedbacks that
favor shrub growth. Summers drier than three-quarters of current rainfall
decreased grass seedling recruitment to negligible values (Peters et al.,
2010). Management cannot reliably increase carbon uptake in arid and
semiarid rangelands, which is most often controlled by abiotic factors
not easily changed by management of grazing or vegetation (Booker
et al., 2013).
Other factors being equal, grasslands and shrublands in cool areas are
expected to respond to warming with increased primary production,
while those in hot areas are expected to show decreased production
(limited evidence, low agreement). A shift to more woody vegetation
states expected to occur (locally but not globally) in tropical grasslands
of the African continent (Higgins and Scheiter, 2012). The response to
warming and drought depends on site, year, and plant species, as shown
by manipulation experiments (Peñuelas et al., 2004, 2007; Gao and
Giorgi, 2008; Grime et al., 2008; Shinoda et al., 2010; Wu and Chen,
2013). In most temperate and Arctic regions, the capacity to support
richer (i.e., more diverse) communities is projected to increase with rising
temperature, while decreases in water availability suggest a decline in
capacity to support species-rich communities in most tropical and
subtropical regions (Sommer et al., 2010). Warming may cause an
asymmetrical response of soil carbon and nitrogen cycles, causing
nitrogen limitation that reduces acclimation in plant production (Beier
et al., 2008).
Some grasslands are exposed to elevated levels of nitrogen deposition,
which alters species composition, increases primary production up to a
point, and decreases it thereafter (see Section 4.2.4.2; Bobbink et al.,
2010; Cleland and Harpole, 2010; Gaudnik et al., 2011). In a study of
162 plots over 25 years, nitrogen deposition drove grassland composition
at the local scale, in interaction with climate, whereas climate changes
were the predominant driver at the regional scale (Gaudnik et al., 2011).
Nitrogen mineralization in shrublands under either arid or wet conditions
is more sensitive to periodic droughts than systems under more mesic
conditions (Emmett et al., 2004). Decreased tissue concentrations of
phosphorus were also associated with warming and drought (Peñuelas et
al., 2004, 2012; Beier et al., 2008). Strong interactions between warming
and disturbances have been observed, leading to increased nitrogen
leaching from shrubland ecosystems (Beier et al., 2004).
Most grasslands and shrublands are characterized by relatively frequent
but low-intensity fires, which affect their plant species composition and
demographics (e.g., Gibson and Hulbert, 1987; Gill et al., 1999; Uys et
al., 2004; de Torres Curth et al., 2012). Species composition changes
may be as important in determining ecosystem impacts as the direct
effects of climate on plant (Suttle et al., 2007). Fire frequency, duration,
and intensity are influenced primarily by climate and secondarily by
management (Pitman et al., 2007; Lenihan et al., 2008; Archibald et al.,

312
Chapter 4 Terrestrial and Inland Water Systems
4
2
009; Giannakopoulos et al., 2009; Armenteras-Pascual et al., 2011),
and are therefore sensitive to climate change; the duration of the fire
season is also projected to broaden (Clarke et al., 2013). Changes in fire
frequency may interact with changes in rainfall seasonality: for instance,
if fires are followed by rainy spring periods in northwestern Patagonia,
as occurs with more frequent El Niño-Southern Oscillation (ENSO)
phenomena, there are more recruitment windows for shrubs (Ghermandi
et al., 2010). Relatively little is known regarding the combined effect of
climate change and increased grazing by large mammals, or on the
consequences for pastoral livelihoods that depend on rangelands
(Thornton et al., 2009).
4.3.3.2.3. Deserts
The deserts of the world, defined as land areas with an arid or hyperarid
climate regime, occupy 35% of the global land surface. Species
composition in desert areas is expected to shift in response to climate
warming (Ooi et al., 2009; Kimball et al., 2010). Deserts are sparsely
populated, but the people who do live there are among the poorest
in the world (Millennium Ecosystem Assessment, 2005a). There is
medium agreement but limited evidence that the present extent of
deserts will increase in the coming decades, despite the projected
increase in rainfall at a global scale, as a result of the strengthening
of the Hadley Circulation, which determines the location of the broad
band of hot deserts approximately 15°N to 30°N and 15°S to 30°S of
the equator (Mitas and Clement, 2005; Seidel et al., 2008; Johanson
and Fu, 2009; Lu et al., 2009; Zhou et al., 2011). There may be a
feedback to the global climate from an increase in desert extent, which
differs in sign between deserts closer to the equator than 20° and those
closer to the pole: in model simulations, extension of the near-equator
“hot deserts” causes warming, while extension of the near-boreal
“cold deserts” causes cooling, in both cases largely through albedo-
mediated effects (Alkama et al., 2012). Deserts are expected to become
warmer and drier at faster rates than other terrestrial regions (Lapola
et al., 2009; Stahlschmidt et al., 2011). Most deserts are already
extremely hot, and therefore further warming likely to be physiologically
injurious rather than beneficial. The ecological dynamics in deserts are
rainfall event-driven (Holmgren et al., 2006), often involving the
concatenation of a number of quasi-independent events. Some desert
tolerance mechanisms (e.g., biological adaptations by long-lived taxa)
may be outpaced by global climate change (Lapola et al., 2009;
Stahlschmidt et al., 2011).
4.3.3.2.4. Mediterranean-type ecosystems
Mediterranean-type ecosystems occur on most continents, and are
characterized by cool, wet winters and hot, dry summers. They were
identified as being among the most likely to be impacted by climate
change in AR4 and received extensive coverage (Fischlin et al., 2007).
Since then, further evidence has accumulated of climate risks to these
systems from rising temperature (Giorgi and Lionello, 2008), rainfall
change (declining in most but not all cases), increased drought (Sections
23.2.3, 25.2), and increased fire frequency (Section 23.4.4). There have
been observed shifts in phenology (Gordo and Sanz, 2010), range
contraction of Mediterranean species (Pauli et al., 2012), declines in the
h
ealth and growth rate of dominant tree species (Allen, C.D. et al., 2010;
Sarris et al., 2011; Brouwers et al., 2012; see also Section 23.4.4), and
increased risk of erosion and desertification, especially in very dry areas
(Lindner et al., 2010; Shakesby, 2011). Model projections show further
species range contractions in the 21st century under all climate change
scenarios. This will result in losses of biodiversity (medium confidence)
(Maiorano et al., 2011; Kuhlmann et al., 2012; see also Sections 23.6.4,
25.1).
4.3.3.3. Rivers, Lakes, Wetlands, and Peatlands
Freshwater ecosystems are considered to be among the most
threatened on the planet (Dudgeon et al., 2006; Vörösmarty et al., 2010).
Fragmentation of rivers by dams and the alteration of natural flow
regimes have led to major impacts on freshwater biota (Pringle, 2001;
Bunn and Arthington, 2002; Nilsson et al., 2005; Reidy Liermann et al.,
2012). Floodplains and wetland areas have become occupied for intensive
urban and agricultural land use to the extent that many are functionally
disconnected from their rivers (Tockner et al., 2008). Pollution from cities
and agriculture, especially nutrient loading, has resulted in declines in
water quality and the loss of essential ecosystem services (Allan, 2004).
As a direct consequence of these and other impacts, freshwaters have
some of the highest rates of extinction of any ecosystem for those
species groups assessed for the IUCN Red List (estimated as much as
4% per decade for some groups, such as crayfish, mussels, fishes, and
amphibians in North America) (Dudgeon et al., 2006), with estimates that
roughly 10,000 to 20,000 freshwater species are extinct or imperilled
as a consequence of human activity (Strayer and Dudgeon, 2010). This
is a particular concern given that freshwater habitats support 6% of all
described species (Dudgeon et al., 2006), including approximately 40%
of the world’s fish diversity and a third of the vertebrate diversity (Balian
et al., 2008).
It is very likely that these stressors to freshwater ecosystems will
continue to dominate as human demand for water resources grows,
accompanied by increased urbanization and expansion of irrigated
agriculture (Vörösmarty et al., 2000; Malmqvist et al., 2008; Dise, 2009).
However, climate change will have significant additional impacts (high
confidence), from altered thermal regimes, altered precipitation and
flow regimes, and, in the case of coastal wetlands, sea level rise. Specific
aquatic habitats that are most vulnerable to these direct climate effects,
especially rising temperatures, are those at high altitude and high latitude,
including Arctic and sub-Arctic bog communities on permafrost, and alpine
and Arctic streams and lakes (see Section 4.3.3.4; Klanderud and Totland,
2005; Smith et al., 2005; Smol and Douglas, 2007b). It is noteworthy
that these high-latitude systems currently experience a relatively low
level of threat from other human activities (Vörösmarty et al., 2010). It
is likely that the shrinkage and disappearance of glaciers will lead to
the reduction of local and regional freshwater biodiversity, with 11 to
38% of the regional macroinvertebrate species pool expected to be lost
following complete disappearance of glaciers (Jacobsen et al., 2012;
Box CC-RF). Shrinkage of glaciers and the loss of small glaciers will
most likely reduce beta diversity at the species and the genetic level, as
predicted for the Pyrenees (Finn et al., 2013). Dryland rivers and wetlands,
many already experiencing severe water stress from human consumptive
use, are also likely to be further impacted by decreased and more variable

313
Terrestrial and Inland Water Systems Chapter 4
4
p
recipitation and higher temperatures. Headwater stream systems in
general are also vulnerable to the effects of warming because their
temperature regimes closely track air temperatures (Caissie, 2006).
There is widespread evidence of rising stream and river temperatures
over the past few decades (Langan et al., 2001; Morrison et al., 2002;
Webb and Nobilis, 2007; Chessman, 2009; Ormerod, 2009; Kaushal et
al., 2010; van Vliet et al., 2011; Markovic et al., 2013; but see Arismendi
et al., 2012). Rising water temperature has been linked by observational
and experimental studies to shifts in invertebrate community composition,
including declines in cold stenothermic species (Brown et al., 2007;
Durance and Ormerod, 2007; Chessman, 2009; Ormerod, 2009). Rising
temperature is also implicated in species range shifts (e.g., Comte and
Grenouillet, 2013), implying changes in the composition of river fish
communities (Daufresne and Boet, 2007; Buisson et al., 2008; Comte
et al., 2013), especially in headwater streams where species are more
sensitive to warming (e.g., Buisson and Grenouillet, 2009).
Rising temperatures in the well-mixed surface waters in many temperate
lakes, resulting in reduced periods of ice formation (Livingstone and
Adrian, 2009; Weyhenmeyer et al., 2011) and earlier onset and increased
duration and stability of the thermocline during summer (Winder and
Schindler, 2004), are projected to favor a shift in dominance to smaller
phytoplankton (Parker et al., 2008; Winder et al., 2009; Yvon-Durocher
et al., 2011) and cyanobacteria (Wiedner et al., 2007; Jöhnk et al., 2008;
Paerl et al., 2011), especially in those ecosystems experiencing high
anthropogenic loading of nutrients (Wagner and Adrian, 2009); with
impacts to water quality, food webs, and productivity (O’Reilly et al.,
2003; Verburg et al., 2003; Gyllström et al., 2005; Parker et al., 2008;
Shimoda et al., 2011). Prolonged stratification and associated anaerobic
conditions near the sediment-water interface can increase the internal
loading of phosphorus, particularly in eutrophic lakes (Søndergaard et
al., 2003; Wilhelm and Adrian, 2008; Wagner and Adrian, 2009).
In many freshwater ecosystems, the input of dissolved organic carbon
through runoff from the catchment has increased, inducing changes in
water color (Hongve et al., 2004; Evans et al., 2005; Erlandsson et al.,
2008). Soil recovery from acidification and changed hydrological
conditions (partly linked to increased precipitation) appear to be the
main factors driving this development (Evans et al., 2005; Monteith et
al., 2007). The resulting increased light attenuation can lead to lower
algal concentrations and loss of submersed vegetation (Ask et al., 2009;
Karlsson et al., 2009).
Emergent aquatic macrophytes are likely to expand their northward
distribution and percentage cover in boreal lakes and wetlands, posing
an increasing overgrowth risk for sensitive macrophyte species (Alahuhta
et al., 2011). Long-term shifts in macroinvertebrate communities have
also been observed in European lakes where temperatures have
increased (Burgmer et al., 2007), noting that warming may increase
species richness in smaller temperate water bodies, especially those at
high altitude (Rosset et al., 2010). Although less studied, it has been
proposed that tropical ectothermic (“cold blooded”) organisms will be
particularly vulnerable because they will approach critical maximum
temperatures proportionately faster than species in high-latitude
environments, despite lower rates of warming (Deutsch et al., 2008;
Hamilton, 2010; Laurance et al., 2011).
T
here is growing evidence that climate-induced changes in precipitation
will significantly alter ecologically important attributes of hydrologic
regimes in rivers and wetlands, and exacerbate impacts from human
water use in developed river basins (high confidence in detection, medium
confidence in attribution; see Box CC-RF; Xenopoulos et al., 2005;
Aldous et al., 2011). Freshwater ecosystems in Mediterranean-montane
ecoregions (e.g., Australia, California, and South Africa) are projected
to experience a shortened wet season and prolonged, warmer summer
season (Klausmeyer and Shaw, 2009), increasing the vulnerability of
fish communities to drought (Magalhães et al., 2007; Hermoso and
Clavero, 2011) and floods (Meyers et al., 2010). Shifts in hydrologic
regimes in snowmelt systems, including earlier runoff and declining base
flows in summer (Stewart et al., 2005; Stewart, 2009), are projected to
alter freshwater ecosystems, through changes in physical habitat and
water quality (Bryant, 2009). Declining rainfall and increased interannual
variability will most likely increase low-flow and dry-spell duration in
dryland regions, leading to reduced water quality in remnant pools
(Dahm et al., 2003), reduction in floodplain egg and seed banks (Capon,
2007; Jenkins and Boulton, 2007), the loss of permanent aquatic refugia
for fully aquatic species and water birds (Johnson et al., 2005; Bond et
al., 2008; Sheldon et al., 2010), altered freshwater food webs (Ledger
et al., 2013), and drying out of wetlands (Davis, J.L. et al., 2010).
Climate-induced changes in precipitation will probably be an important
factor altering peatland vegetation in temperate and boreal regions, with
decreasing wetness during the growing season generally associated with
a shift from a Sphagnum dominated to vascular plant dominated
vegetation type and a general decline of carbon sequestration in the
long term (Limpens et al., 2008). Mire ecosystems (i.e., bogs, transition
bogs, and fens) in central Europe face severe climate-induced risk, with
increased summer temperatures being particularly important (Essl et al.,
2012). Decreased dry season precipitation and longer dry seasons in
major tropical peatland areas in Southeast Asia are projected to result
in lower water tables more often and for longer periods, with an increased
risk of fire (Li et al., 2007; Rieley et al., 2008; Frolking et al., 2011).
Peatlands contain large stocks of carbon that are vulnerable to change
through land use and climate change. Although peatlands cover only
about 3% of the land surface, they hold the equivalent of half of the
atmosphere’s carbon (as CO
2
), or one-third of the world’s soil carbon
stock (400 to 600 Pg) (Limpens et al., 2008; Frolking et al., 2011; Page
et al., 2011). About 14 to 20% of the world’s peatlands are currently
used for agriculture (Oleszczuk et al., 2008) and many, particularly
peat swamp forests in Southeast Asia, are undergoing rapid major
transformations through drainage and burning in preparation for oil
palm and other crops or through unintentional burning (Limpens et al.,
2008; Hooijer et al., 2010). Deforestation, drainage, and burning in
Indonesian peat swamp forests can release 59.4 ± 10.2 Mg CO
2
ha
–1
yr
–1
over 25 years (Murdiyarso et al., 2010), contributing significantly
to global GHG emissions, especially during periods of intense drought
associated with ENSO when burning is more common (Page et al.,
2002). Anthropogenic disturbance has changed peatlands from being
a weak global carbon sink to a source (Frolking et al., 2011), though
interannual variability is large. Fluvial export can also be a significant
contributor to carbon losses that has been largely overlooked to date,
with recent estimates of DOC export from degraded tropical peatlands
50% higher than in intact systems (Moore et al., 2013). Conserving

314
Chapter 4 Terrestrial and Inland Water Systems
4
p
eatland areas not yet developed for biofuels or other crops, or
rewetting and restoring degraded peatlands to preserve their carbon
store, are potential mitigation strategies.
Sea level rise will lead to direct losses of coastal wetlands with associated
impacts on water birds and other wildlife species dependent on fresh
water (BMT WBM, 2010; Pearlstine et al., 2010; Traill et al., 2010), but
the impact will probably be relatively small compared with the degree
of direct and indirect human-induced destruction (Nicholls, 2004). River
deltas and associated wetlands are particularly vulnerable to rising sea
level, and this threat is further compounded by trapping of sediment in
reservoirs upstream and subsidence from removal of oil, gas, and water
(Syvitski et al., 2009; see Section 5.4.2.7). Lower river flows might
exacerbate the impact of sea level rise and thus salinization on freshwater
ecosystems close to the ocean (Ficke et al., 2007).
4.3.3.4. Tundra, Alpine, and Permafrost Systems
The High Arctic region, with tundra-dominated landscapes, has warmed
more than the global average over the last century (Kaufman et al.,
2009; see WGI AR5 Chapter 2). Changes consistent with warming are
evident in the freshwater and terrestrial ecosystems and permafrost of
the region (Hinzman et al., 2005; Axford et al., 2009; Jia, G.J. et al., 2009;
Post et al., 2009; Prowse and Brown, 2010; Romanovsky et al., 2010;
Walker et al., 2012). Most of the Arctic has experienced recent change
in vegetation photosynthetic capacity, particularly adjacent to rapidly
retreating sea ice (Bhatt et al., 2010). Changes in terrestrial environments
in Antarctica have also been reported. Vieira et al. (2010) show that in
in the Maritime Antarctic permafrost temperatures are close to thaw.
Permafrost warming has been observed in continental Antarctica
(Guglielmin and Cannone, 2012) and for the Palmer archipelago
(Bockheim et al., 2013).
Continued warming is projected to cause the terrestrial vegetation and
lake systems of the Arctic to change substantially (high confidence).
Continued expansion in woody vegetation cover in tundra regions over
the 21st century is projected by the CMIP5 ESMs (Bosio et al., 2012; see
WGI AR5 Chapter 6), by dynamic global vegetation models driven by
other climate model projections, and by observationally based statistical
models (Pearson et al., 2013). Changes may be complex (see Box 4-4)
and in some cases involve nonlinear and threshold responses to warming
and other climatic change (Hinzman et al., 2005; Mueller, D.R. et al., 2009;
Bonfils et al., 2012). Arctic vegetation change is expected to continue
long after any stabilization of global mean temperature (see WGI AR5
Chapter 6; Falloon et al., 2012). In some regions, reduced surface albedo
due to increased vegetation cover is projected to cause further local
warming even in scenarios of stabilized GHG concentrations (Falloon
et al., 2012).
In the Arctic tundra biome (in contrast to the boreal forests discussed
in Section 4.3.3.1.1), vegetation productivity has systematically
increased over the past few decades in both North America and
northern Eurasia (Goetz et al., 2007; Stow et al., 2007; Jia, G.J. et al.,
2009; de Jong et al., 2011; Myers-Smith et al., 2011; Elmendorf et al.,
2012). This phenomenon is amplified by retreat of coastal sea ice (Bhatt
et al., 2010) and has been widely discussed in the context of increased
s
hrub growth and expansion over the last half century (Forbes et al.,
2010; Myers-Smith et al., 2011). Deciduous shrubs and graminoids
respond to warming with increased growth (Walker, 2006; Epstein et
al., 2008; Euskirchen et al., 2009; Lantz et al., 2010). Analyses of satellite
time series data show the increased productivity trend is not unique to
shrub-dominated tundra areas (Jia, G.J. et al., 2009; Beck and Goetz,
2011); thus greening is a response shared by multiple vegetation
communities and continued changes in the tundra biome can be
expected irrespective of shrub presence. The very large spatial scale over
which these changes are occurring, the strong warming signal over
much of the Arctic for the last 5 decades (Burrows et al., 2011), and the
absence of strong confounding factors means that detection of these
changes in Arctic systems and their attribution to global warming can
be made with high confidence, despite the relatively short time frame
of most observations (Figure 4-4).
Shrub expansion and height changes are particularly important because
they trap snow, mediate winter soil temperature and summer moisture
regimes, increase nutrient mineralization, and produce a positive feedback
for additional shrub growth (Sturm et al., 2005; Lawrence et al., 2007;
Bonfils et al., 2012). Although increased shrub cover and height produce
shadowing that reduce ground heat flux and active layer depth, they also
reduce surface albedo, increase energy absorption and evapotranspiration
(Chapin III et al., 2005; Blok et al., 2010), and produce feedbacks that
reinforce shrub densification and regional warming (Lawrence and
Swenson, 2011; Bonfils et al., 2012). On balance, these feedbacks can
act to partially offset one another, but when coupled with warmer and
wetter conditions they act to increase active layer depth and permafrost
thaw (Yi et al., 2007; Bonfils et al., 2012).
The Arctic tundra biome is experiencing increasing fire disturbance and
permafrost degradation. Both of these processes facilitate conditions
for woody species establishment in tundra areas, either through
incremental migration or via more rapid long-distance dispersal to areas
reinitialized by burning (Epstein et al., 2007; Goetz et al., 2011). When
already present at the boreal-tundra ecotone, shrub and tree species
show increased productivity with warmer conditions (Devi et al., 2008;
Andreu-Hayles et al., 2011; Elmendorf et al., 2012). Tundra fires not only
emit large quantities of combusted carbon formerly stored in vegetation
and organic soils (Mack et al., 2011; Rocha and Shaver, 2011), but also
increase active layer depth during summer months (Racine et al., 2004;
Liljedahl et al., 2007; Jorgenson et al., 2010), produce landforms
associated with thawing of ice-rich permafrost, and can create conditions
that alter vegetation succession (Racine et al., 2004; Lantz et al., 2009;
Higuera et al., 2011).
It is virtually certain that the area of NH permafrost will continue to
decline over the first half of the 21st century (see WGI AR5 Chapter 12)
in all RCP scenarios (Figure 4-9; Caesar et al., 2013; Koven et al., 2013).
In the RCP2.6 scenario of an early stabilization of CO
2
concentrations,
the permafrost area is projected to stabilize at a level approximately 20%
below the 20th century area, and then begin a slight recovering trend. In
RCP4.5, in which CO
2
concentration is stabilized at approximately 550
ppmv by the mid-21st century, the simulations that extend beyond 2100
show permafrost continuing to decline for at least another 250 years.
In the RCP8.5 scenario of ongoing CO
2
rise, the permafrost area is
simulated to approach zero by the middle of the 22nd century in
315
Terrestrial and Inland Water Systems Chapter 4
4
simulations that extend beyond 2100. RCP8.5 simulations that ended
at 2100 showed continued permafrost decline in the late 21st century,
although at slower rates in some cases as the remaining permafrost
area decreases (Figure 4-9.).
Frozen soils and permafrost currently hold about 1700 PgC, more than
twice the carbon than the atmosphere, and thus represent a particularly
large vulnerability to climate change (i.e., warming) (see WGI AR5
Chapter 6). Although the Arctic is currently a net carbon sink, continued
warming will act to turn the Arctic to a net carbon source, which will in
turn create a potentially strong positive feedback to accelerate Arctic
(and global) warming with additional releases of CO
2
, CH
4
, and perhaps
N
2
O, from the terrestrial biosphere into the atmosphere (high confidence;
Schuur et al., 2008, 2009; Maslin et al., 2010; McGuire et al., 2010;
O’Connor et al., 2010; Schaefer et al., 2011; see WGI AR5 Chapter 6 for
detailed treatment of biogeochemistry, including feedbacks). Moreover,
this feedback is already accelerating due to climate-induced increases
in fire (McGuire et al., 2010; O’Donnell et al., 2011). The rapid retreat
of snow cover and resulting spread of shrubs and trees into areas
currently dominated by tundra has begun, and will continue to serve
1900 2000 22002100 2300
Permafrost area (10
6
km
2
)
30
25
20
15
10
5
0
1900 2000 22002100 2300
Permafrost area (10
6
km
2
)
30
25
2
0
1
5
10
5
0
1
900 2000 22002100 2300
Permafrost area (10
6
km
2
)
30
2
5
2
0
1
5
10
5
0
HadGEM2-ES
IPSL-CM5A-LR
IPSL-CMA5A-MR
MICROC-ESM-CHEM
MICROC-ESM
MICROC5
MPI-ESM-LR
MRI-CGCM3
BCC-CSM1-1
INM-CM4
NorESM1-M
(a) RCP2.6 modeled permafrost extent
(b) RCP4.5 modeled permafrost extent
(c) RCP8.5 modeled permafrost extent
CCSM4
CESM1-CAM5
CanESM2
GFDL-ESM2G
GFDL-ESM2G
GISS-E2-R
HadCM3
HadGEM2-CC
Figure 4-9 | CMIP5 multi-model simulated area of Northern Hemisphere permafrost in the upper 3 m of soil, from 1850 to 2100 or 2300 depending on extent of individual
simulations. Each panel shows historical (1850–2005) and projected (2005–2100 or 2300) simulations for (a) Representative Concentration Pathway 2.6 (RCP2.6), (b) RCP4.5,
and (c) RCP8.5. The observed current permafrost extent is 15 × 10
6
km
2
. (Based on Koven et al., 2013, with analysis extended to 2300 following Caesar et al., 2013).
316
Chapter 4 Terrestrial and Inland Water Systems
4
Box 4-4 | Boreal-Tundra Biome Shift
Changes in a suite of ecological processes currently underway across the broader Arctic region are consistent with Earth System
Model (ESM) predictions of climate-induced geographic shifts in the range extent and functioning of the tundra and boreal forest
biomes (Figure 4-10). Until now, these changes have been gradual shifts across temperature and moisture gradients, rather than
abrupt. Responses are expressed through gross and net primary production, microbial respiration, fire and insect disturbance,
vegetation composition, species range expansion and contraction, surface energy balance and hydrology, active layer depth and
permafrost thaw, and a range of other inter-related variables. Because the high northern latitudes are warming more rapidly than
other parts of the Earth, due at least in part to Arctic amplification (Serreze and Francis, 2006), the rate of change in these ecological
processes are sufficiently rapid that they can be documented in situ (Hinzman et al., 2005; Post et al., 2009; Peng et al., 2011;
Elmendorf et al., 2012) as well as from satellite observations (Goetz et al., 2007; Beck, P.S.A. et al., 2011; Xu et al., 2013) and captured
in ESMs (McGuire et al., 2010).
ConiferDead treeShrub Fire
Tundra
Boreal
forest
Coastal
sea
Sea ice
B
i
o
m
e
s
m
o
v
i
n
g
n
o
r
t
h
Gap opening between land and
sea ice alters coastal circulation
Decrease
in albedo
Shorter duration
of snow cover
Shrub
encroachment
and densification
Warmer spring
and summer
Increased fire
intensity
CO
2
emissions
Replacement
of conifers by
broadleaf forest
Sea ice retreating
Insulating organic layer contains a large
carbon stock that decomposes faster
under warmer climate
Lakes drain or form as permafrost
thaws and drainage changes
New wetlands emit CH
4
Permafrost is thawing in warmer regions
Permafrost
Organic layer
Soil carbon export to rivers
increases with permafrost thaw
Global
warming
Figure 4-10 | Tundra–boreal biome shift. Earth System Models predict a northward shift of Arctic vegetation with climate warming, as the boreal biome migrates into
what is currently tundra. Observations of shrub expansion in tundra, increased tree growth at the tundra–forest transition, and tree mortality at the southern extent of
the boreal forest in recent decades are consistent with model projections. Vegetation changes associated with a biome shift, which is facilitated by intensification of the
fire regime, will modify surface energy budgets, and net ecosystem carbon balance, permafrost thawing, and methane emissions, with net feedbacks to additional
climate change.
Continued next page

317
Terrestrial and Inland Water Systems Chapter 4
4
as a positive feedback accelerating high-latitude warming (Chapin III
et al., 2005; Bonfils et al., 2012).
There is medium confidence that rapid change in the Arctic is affecting
its animals. For example, seven of 19 sub-populations of the polar bear
are declining in number, while four are stable, one is increasing, and the
remaining seven have insufficient data to identify a trend (Vongraven
and Richardson, 2011). Declines of two of the sub-populations are
linked to reductions in sea ice (Vongraven and Richardson, 2011). Polar
bear populations are projected to decline greatly in response to
continued Arctic warming (Hunter et al., 2010; Stirling and Derocher,
2012), and it is expected that the populations of other Arctic animals
will be affected dramatically by climate change, often in complex but
potentially dramatic ways (e.g., Post et al., 2009; Sharma et al., 2009;
Gallant et al., 2012; Gilg et al., 2012; Post and Brodie, 2012; Gauthier
et al., 2013; Nielsen and Wall, 2013; Prost et al., 2013; White et al.,
2013). Simple niche-based or climatic envelope models have difficulty
in capturing the full complexity of these future changes (MacDonald,
2010).
There is high confidence that alpine systems are already showing a high
sensitivity to ongoing climate change and will be highly vulnerable to
change in the future. In western North America, warming, glacier retreat,
snowpack decline, and drying of soils are already causing a large increase
in mountain forest mortality and wildfire, plus other ecosystem impacts
(e.g., Westerling et al., 2006; Crimmins et al., 2009; van Mantgem et al.,
2009; Pederson et al., 2010; Muhlfeld et al., 2011; Brusca et al., 2013;
Williams et al., 2013), and disturbance will continue to be an important
agent of climate-induced change in this region (Littell et al., 2010).
Globally, tree line altitude appears to be changing, although not always
in simple ways (Harsch et al., 2009; Tingley et al., 2012) and may
sometimes be due to factors not related to climate change. Responses
to climate change in high-altitude ecosystems are taking place in Africa,
Asia, Europe, and elsewhere (Cannone et al., 2007, 2008; Yasuda et al.,
2007; Lenoir et al., 2008, 2010; Britton et al., 2009; Chen et al., 2009,
2011; Cui and Graf, 2009; Normand et al., 2009; Allen, C.D. et al., 2010;
Eggermont et al., 2010; Engler et al., 2011; Kudo et al., 2011; Laurance
et al., 2011; Dullinger et al., 2012). For example, in a study of permanent
plots from 1994 to 2004 in the Austrian high Alps, a range contraction
of subnival to nival plant species was indicated at the downslope edge,
and an expansion of alpine pioneer species at the upslope edge (Pauli
et al., 2007). Thermophilous vascular plant species were observed to
colonize in alpine mountain-top vegetation across Europe during the
past decade (Gottfried et al., 2012). As with the Arctic, permafrost
thawing in alpine systems could provide a strong positive feedback (e.g.,
Tibet; Cui and Graf, 2009).
4.3.3.5. Highly Human-Modified Systems
About a quarter of the land surface is now occupied by ecosystems
highly modified by human activities. In this section we assess the
vulnerability to climate change only of those modified systems not dealt
with elsewhere, that is, excluding agriculture (Chapter 7), freshwater
fisheries (Chapter 3), and urban areas (Chapter 8).
4.3.3.5.1. Plantation forestry
Plantation forests are established through afforestation or reforestation,
often with tree crop replacement (Dohrenbusch and Bolte, 2007; FAO,
2010). They differ from natural or semi-natural forests (Section 4.3.3.1)
by generally being even-aged, having a reduced species diversity
(sometimes of non-native species), and being dedicated to the production
of timber, pulp, and/or bioenergy. Plantation forests contribute 7% to
the global forest area (FAO, 2010), an increase of 5 million hectares
between 2000 and 2010 (FAO, 2010). Most recent plantations have
been established by afforestation of non-forest areas in the tropics and
subtropics and some temperate regions, particularly China (Kirilenko
and Sedjo, 2007; FAO, 2010). Afforestation usually results in net CO
2
uptake from the atmosphere (Canadell and Raupach, 2008; Van Minnen
et al., 2008) but does not necessarily result in a reduction in global
warming (Bala et al., 2007; see Section 4.3.4.5).
Growth rates in plantation forests have generally increased during the
last decades but the variability is large. In forests that are not highly
Box 4-4 (continued)
Gradual changes in composition resulting from decreased evergreen conifer productivity and increased mortality, as well as increased
deciduous species productivity, can be facilitated by more rapid shifts associated with fire disturbance where it can occur (Mack et al.,
2008; Johnstone et al., 2010; Roland et al., 2013). Each of these interacting processes, as well as insect disturbance and associated
tree mortality, are tightly coupled with warming-induced drought (Choat et al., 2012; Ma et al., 2012; Anderegg et al., 2013a). Similarly,
gradual productivity increases at the boreal-tundra ecotone are facilitated by long distance dispersal into areas disturbed by tundra
fire and thermokarsting (Tchebakova et al., 2009; Brown, 2010; Hampe, 2011). In North America these coupled interactions set the
stage for changes in ecological processes, already documented, consistent with a biome shift characterized by increased deciduous
composition in the interior boreal forest and evergreen conifer migration into tundra areas that are, at the same time, experiencing
increased shrub densification. The net feedback of these ecological changes to climate is multi-faceted, complex, and not yet well
known across large regions except via modeling studies, which are often poorly constrained by observations.

318
Chapter 4 Terrestrial and Inland Water Systems
4
w
ater limited, increased growth is consistent with higher temperatures
and extended growing seasons. As in the case of forests in general, clear
attribution is difficult because of the interaction of multiple environmental
drivers as well as changes in forest management (e.g., Boisvenue and
Running, 2006; Ciais et al., 2008; Dale et al., 2010; see also Section
4.3.3.1). In Europe much of the increase has been attributed to recovery
following previously more intense harvesting (Ciais et al., 2008; Lindner
et al., 2010).
Several studies using forest yield models suggest future increases in
forest production (Kirilenko and Sedjo, 2007). These results may
overestimate the positive effects of elevated CO
2
(Kirilenko and Sedjo,
2007; see Section 4.2.4.4). The effects of disturbances such as wildfires,
forest pests, pathogens, and windstorms, which are major drivers of
forest dynamics, are poorly represented in the models (Loustau, 2010;
see also Section 4.3.3.1 and Box 4-2). The results from different models
often differ substantially both regarding forest productivity (e.g., Sitch
et al., 2008; Keenan et al., 2011) and potential species ranges (see
Section 4.3.3.1.2). Decreased forest production is expected in already
dry forest regions for which further drying is projected, such as the
southwestern USA (Williams, A.P. et al., 2010). Extreme drying may also
decrease yields in forests currently not water limited (e.g., Sitch et al.,
2008; see Section 4.3.3.1). Plantations in cold-limited areas could
benefit from global warming, provided that increased fires, storms,
pests, and pathogens do not outweigh the potential direct climate
effects on tree growth rates.
Low species diversity (and low genetic diversity within species where
clones or selected provenances are used) renders plantation forests less
resilient to climate change than natural forests (e.g., Hemery, 2008).
Choosing provenances that are well adapted to current climates but
pre-adapted to future climates is difficult because of uncertainties in
climate projections at the time scale of a plantation forest rotation
(Broadmeadow et al., 2005). How forest pests and pathogens will spread
as a result of climate change and other factors is highly uncertain. New
pathogen-tree interactions may arise (e.g., Brasier and Webber, 2010).
Adaptive management can decrease the vulnerability of plantation
forests to climate change (Hemery, 2008; Bolte et al., 2009; Seppälä,
2009; Dale et al., 2010). For example, risk spreading by promoting mixed
stands, containing multiple species or provenances, combined with
natural regeneration (Kramer et al., 2010), has been advocated as an
adaptation strategy for temperate forests (Hemery, 2008; Bolte et al.,
2010) and tropical forests (Erskine et al., 2006; Petit and Montagnini,
2006). Incomplete knowledge of the ecology of tropical tree species and
little experience in managing mixed tropical tree plantations remains a
problem (Hall et al., 2011). Especially at the equator-ward limits of cold-
adapted species, such as Norway spruce (Picea abies) in Europe, climate
change will very likely lead to a shift in the main tree species used for
forest plantations (Iverson et al., 2008; Bolte et al., 2010).
4.3.3.5.2. Bioenergy systems
The production of modern bioenergy is growing rapidly throughout the
world in response to climate mitigation and energy security policies
(Kirilenko and Sedjo, 2007). WGIII AR5 Chapter 7 addresses the potential
of bioenergy as a climate mitigation strategy. The vulnerability of
b
ioenergy systems to climate change is similar to that of plantation
forestry (Section 4.3.3.5.1) or food crops (Section 7.3): in summary, they
remain viable in the future in most but not all locations, but their
viability is increasingly uncertain for high levels of climate change
(Haberl et al., 2011). Oliver, R.J. et al. (2009) suggested that rising CO
2
might contribute to increased drought tolerance in bioenergy crops
(because it leads to improved plant water use efficiency).
The unintended consequences of large-scale land use changes driven
by increasing bioenergy demand are addressed in Section 4.4.4.
4.3.3.5.3. Cultural landscapes
Cultural landscapes are characterized by a long history of human-nature
interactions, which results in a particular configuration of species and
landscape pattern attaining high cultural significance (Rössler, 2006).
Examples are grassland or mixed agriculture landscapes in Europe, rice
landscapes in Asia (Kuldna et al., 2009), and many others across the globe
(e.g., Rössler, 2006; Heckenberger et al., 2007). Such landscapes are often
agricultural, but we deal with them here because their perceived value is
only partly in terms of their agricultural products.
It has been suggested that protected area networks (such as Natura
2000 in Europe, which includes many cultural landscape elements) be
adjusted to take into account climate change (Bertzky et al., 2010).
Conserving species in cultural landscapes (e.g., EU Council, 1992)
generally depends on maintaining certain types of land use. Doing so
under climate change requires profound knowledge of the systems and
species involved, and conservation success so far has been limited (see
Thomas et al., 2009, for a notable exception). Understanding the relative
importance of climate change and land management change is critical
(Settele and Kühn, 2009). To date land use changes have been the
most obvious driver of change (Nowicki et al., 2007); impacts have been
attributed to climate change (with low to medium confidence) in only
a few examples (Devictor et al., 2012). Even in these, combined land
use-climate effects explain the pattern of observed threats better than
either alone (Schweiger et al., 2008, 2012; Clavero et al., 2011).
There is very high confidence that species composition and landscape
structure are changing in cultural landscapes such as Satoyama
landscapes in Japan or mixed forest, agricultural landscapes in Europe.
Models and experiments suggest that climate change should be
contributing to these observed changes. The land use and land
management signal is so strong in these landscapes that there is very
low confidence that we can attribute these observations to climate
change (Figure 4-4).
4.3.3.5.4. Urban ecosystems
Although urban areas (for definition see Section 8.1.2) cover only 0.5%
of the Earth’s land surface (Schneider et al., 2009), more than half of
humanity lives there (increasing annually by 74 million people; UN DESA
Population Division, 2012) and they harbor a large variety of species
(McKinney, 2008). The frequency and magnitude of warm days and
nights (heat waves) is virtually certain to increase globally in the future

319
Terrestrial and Inland Water Systems Chapter 4
4
(IPCC, 2012); this trend is higher in urban than in rural areas (McCarthy
et al., 2010). Heavy rainfall events are also projected to increase (IPCC,
2012), and although the hydrological conditions in urban areas make
them prone to flooding (medium confidence), there is limited evidence
that they will be over-proportionally affected. It is very likely that sea level
rise in the future will contribute to flooding, erosion, and salinization of
coastal urban ecosystems (IPCC, 2012). Climate change is projected to
increase the frequency of landslides (UN-HABITAT, 2011). Climate
change impacts on urban ecosystems and biodiversity have received
comparatively little attention, with water availability being an exception
(Hunt and Watkiss, 2011). Changes in water availability and quality due
to changes in precipitation, evaporation, or in salinity regimes will
especially affect urban freshwater ecosystems (Hunt and Watkiss, 2011).
As in other ecosystems, climate change will lead to a change in species
composition, the frequency of traits, and ecosystem services from urban
ecosystems. Knapp, S. et al. (2008) found that trait composition of plant
communities changes during urbanization toward adaptive characteristics
of dry and warm environments (see also Sections 4.2.4.6 and 4.3.2.5).
Urban areas are one of the main points of introduction of alien species
(e.g., for plants through urban gardening; Knapp, S. et al., 2012). Increased
damage by phytophagous insects to plants in urban environments is
anticipated (Kollár et al., 2009; Lopez-Vaamonde et al., 2010; Tubby and
Webber, 2010; see also Section 8.2.4.5).
4.3.4. Impacts on Key Ecosystem Services
Ecosystem services are the benefits that people derive from ecosystems
(see Glossary). Many ecosystem services are plausibly vulnerable to
climate change. The Millennium Ecosystem Assessment classification
(Millennium Ecosystem Assessment, 2003) recognizes provisioning
services such as food (Chapter 7), fiber (Section 4.3.4.2), bioenergy
(Section 4.3.4.3), and water (Chapter 3); regulating services such as
climate regulation (Section 4.3.4.5), pollination, pest and disease control
(Section 4.3.4.4), and flood control (Chapter 3); supporting services such
as primary production (Section 4.3.2.2) and nutrient cycling (Section
4.2.4.2, and indirectly Section 4.3.2.3); and cultural services, including
recreation and aesthetic and spiritual benefits (Section 10.6). Section
4.3.4.1 focuses on ecosystem services not already covered in the sections
referenced above.
4.3.4.1. Habitat for Biodiversity
Climate change can alter habitat for species by inducing (1) shifts in
habitat distribution that are not followed by species, (2) shifts in species
distributions that move them outside of their preferred habitats, and
(3) changes in habitat quality (Dullinger et al., 2012; Urban et al., 2012).
Climate change impacts on habitats for biodiversity are already occurring
(see the polar bear example in Section 28.2.2.1.3) but are not yet a
widespread phenomenon. Models of future climate change-induced
shifts in the distribution of ecosystems suggest that many species could
be outside of their preferred habitats within the next few decades
(Urban et al., 2012; see Sections 4.3.2.5, 4.3.3, and Figure 4-1).
Hole et al. (2009) report that the majority of African birds would have
to move large distances (up to several hundred kilometers) over the
next 60 years (under SRES B2a), resulting in substantial turnover of
species within protected areas (>50% turnover in more than 40% of
Important Bird Areas of Africa). To reach suitable climates they will have
to migrate across unfavorable habitats. Many may continue to find
suitable climate within the protected area network, but will be forced
to cope with new habitat constraints (Hole et al., 2009). Araujo et al.
(2011) estimate that by 2080 approximately 60% (58 ± 2.6%) of plants
and vertebrate species will no longer have favorable climates within
European protected areas, often pushing them into unsuitable or less
preferred habitats (based on SRES A1, A2, B1, and A1FI scenarios). Wiens
et al. (2011) project similar effects in the western USA (until the year
2069, based on SRES A2 scenarios), but also find that climate change
may open up new opportunities for protecting species in areas where
Frequently Asked Questions
FAQ 4.5 | Why does it matter if ecosystems are altered by climate change?
Ecosystems provide essential services for all life: food, life-supporting atmospheric conditions, drinkable water, as
well as raw materials for basic human needs such as clothing and housing. Ecosystems play a critical role in limiting
the spread of human and non-human diseases. They have a strong impact on the weather and climate itself, which
in turn impacts agriculture, food supplies, socioeconomic conditions, floods, and physical infrastructure. When
ecosystems change, their capacity to supply these services changes as well—for better or worse. Human well-being
is put at risk, along with the welfare of millions of other species. People have a strong emotional, spiritual, and
ethical attachment to the ecosystems they know, and the species they contain.
By “ecosystem change” we mean changes in some or all of the following: the number and types of organisms present;
the ecosystem’s physical appearance (e.g., tall or short, open or dense vegetation); and the functioning of the
system and all its interactive parts, including the cycling of nutrients and productivity. Though in the long term not
all ecosystem changes are detrimental to all people or to all species, the faster and further ecosystems change in
response to new climatic conditions, the more challenging it is for humans and other species to adapt to the new
conditions.

320
Chapter 4 Terrestrial and Inland Water Systems
4
c
limate is currently unsuitable. In some cases climate change may allow
species to move into areas of lower current or future land use pressure
including protected areas (Bomhard et al., 2005). These studies strongly
argue for a rethinking of protected areas networks and of the
importance of the habitat matrix outside of protected areas as a key to
migration and long-term survival of species (see Sections 4.4.2.2,
4.4.2.3).
In the long term, some habitat types may disappear entirely due to
climate change (see Section 4.3.3 and Figure 4-1). Climates are projected
to occur in the future that at least in some features do not represent
climates that existed in the past (Williams, J.W. et al., 2007; Wiens et
al., 2011), and in the past climate shifts have resulted in vegetation
types that have no current analog (Section 4.2.3). The impacts of habitat
change on species abundance and extinction risk are difficult to evaluate
because at least some species are able to adapt to novel habitats (Prugh
et al., 2008; Oliver, T. et al., 2009). The uncertainty in habitat specificity
is one reason why quantitative projection of changes in extinction rates
is difficult (Malcolm et al., 2006).
The effects of climate change on habitat quality are less well studied
than shifts in species or habitat distributions. Several recent studies
indicate that climate change may have altered habitat quality already
and will continue to do so (Iverson et al., 2011; Matthews et al., 2011).
For example, decreasing snowfall in the southwestern USA has negatively
affected the habitat for songbirds (Martin and Maron, 2012).
4.3.4.2. Timber and Pulp Production
In most areas with forest plantations, forest growth rates have increased
during the last decades, but the variability is large, and in some areas
production has decreased (see Section 4.3.3.1). In forests that are not
highly water limited, these trends are consistent with higher temperatures
and extended growing seasons, but, as in the case of forests in general,
clear attribution is difficult because many environmental drivers and
changes in forest management interact (e.g., Boisvenue and Running,
2006; Ciais et al., 2008; Dale et al., 2010; see also Section 4.3.3.1). In
Europe a reduction in harvesting intensity has contributed (Ciais et al.,
2008; Lindner et al., 2010).
Forest yield models project future increases in forest production under
climate change, perhaps over optimistically (Kirilenko and Sedjo, 2007;
see Section 4.2.4.4). Using a model that accounts for fire effects and
insect damage, Kurz et al. (2008) showed that the Canadian forest sector
may have transitioned from a sink to a source of carbon.
4.3.4.3. Biomass-Derived Energy
Bioenergy sources include traditional forms such as wood and charcoal
from forests (see Section 4.3.3.1) and more modern forms such as the
industrial burning of biomass wastes, the production of ethanol and
biodiesel, and plantations of bioenergy crops. While traditional biofuels
have been in general decline as users switch to fossil fuels or electricity,
they remain dominant energy sources in many less developed parts of
the world, such as Africa, and retain a niche in developed countries.
G
enerally, potentials of bioenergy production under climate change may
be high, but are very uncertain (Haberl et al., 2011).
4.3.4.4. Pollination, Pest, and Disease Regulation
It can be inferred that global change will result in new communities
(Gilman et al., 2010; Schweiger et al., 2010). As these will have had
little opportunity for coevolution, changes in ecological interactions,
such as shifts in herbivore diets, the range of prey of predators, or in
pollination networks are to be expected (Tylianakis et al., 2008; Schweiger
et al., 2012). This may result in temporarily reduced effectiveness of the
“regulating services,” which generally depend on species interactions
(Montoya and Raffaelli, 2010). Burkle et al. (2013) show that the loss
of species reduces co-occurrence of interacting species and thus reduces
ecosystem functions based on them.
Climate change tends to increase the abundance of pest species,
particularly in previously cooler climates, but assessments of changes
in impacts are hard to make (Payette, 2007). Insect pests are directly
influenced by climate change, for example, through a longer warm
season during which to breed, and indirectly, for example, through the
quality of food plants (Jamieson et al., 2012) or via changes in their
natural enemies (predators and parasitoids). Insects have well-defined
temperature optima; warming toward the optimum leads to increased
vitality and reproduction (Allen, C.D. et al., 2010). Mild winters in
temperate areas promote pests formerly controlled by frost sensitivity.
For the vast majority of indirect effects, information is scarce. Further
assessments of climate change effects on pest and disease dynamics
are found in Sections 7.3.2.3 for agricultural pests and 11.5.1 for human
diseases.
Climate change has severe negative impacts on pollinators (including
honeybees) and pollination (Kjøhl et al., 2011) (medium confidence).
After land use changes, climate change is regarded as the second most
relevant factor responsible for the decline of pollinators (Potts et al.,
2010; for other factors see Biesmeijer et al., 2006; Brittain et al.,
2010a,b). The potential influence of climate change on pollination can
be manifold (compare Hegland et al., 2009; Schweiger et al., 2010;
Roberts et al., 2011). There are a few observational studies, which mostly
relate to the phenological decoupling of plants and their pollinators
(Gordo and Sanz, 2005; Bartomeus et al., 2011). While Willmer (2012)
states, based on experimental studies, that phenological effects may be
less important than has been suggested, an analysis of phenological
observations in plants by Wolkovich et al. (2012) shows that experimental
data on phenology may grossly underestimate the actual phenological
shifts.
Le Conte and Navajas (2008) state that the generally observed decline
in honeybees is a clear indication of an increasing susceptibility to
global change phenomena, with pesticide application, new diseases,
and stress (and a combination of these) as the most relevant causes.
Climate change may contribute by modifying the balance between
honeybees and their environment (including exposure or susceptibility
to diseases). Honeybees show a high capacity to adjust to a variety of
environments; their high genetic diversity should allow them to also
cope with climatic change (Bartomeus et al., 2011). The preservation of

321
Terrestrial and Inland Water Systems Chapter 4
4
g
enetic variability within honeybees is regarded as a key adaptation
strategy for pollination services (Le Conte and Navajas, 2008).
4.3.4.5. Moderation of Climate Change, Variability, and Extremes
The focus of this section is on processes operating at regional to global
scales, rather than the well-known microclimatic benefits of ecosystems
in smoothing day-night temperature variations and providing local
evaporative cooling. In the decade 2000–2009, the global net uptake of
CO
2
by terrestrial ecosystems was a large fraction of the anthropogenic
CO
2
emissions to the atmosphere from all sources, reducing the rate of
climate change proportionately (Section 4.3.2.3; WGI AR5 Section 6.3.2).
Afforestation or reforestation are potential climate mitigation options
(Van Minnen et al., 2008; Vaughan and Lenton, 2011; Fiorese and Guariso,
2013; Singh et al., 2013) but, as discussed in Section 4.2.4.1, the net
effect of afforestation on the global climate is mixed and context
dependent. Wickham et al. (2012) found significant positive correlations
between the average annual surface temperature and the proportion
of forest in the landscape and conclude that the climate benefit of
temperate afforestation is unclear. Where low-albedo forest canopies
replace higher-albedo surfaces such as soil, grassland, or snow, the
resultant increase in net radiative forcing counteracts the benefits of
carbon sequestration to some degree (Arora and Montenegro, 2011).
Where the cloud cover fraction is low and the albedo difference is large,
that is, outside the humid tropics, the long-term net result of afforestation
can be global warming (Bala et al., 2007; Bathiany et al., 2010; Schwaiger
and Bird, 2010). Accounting for changes in albedo and indirect greenhouse
effects are not currently required in the formal rules for quantifying for
the climate effects of land use activities (Schwaiger and Bird, 2010;
Kirschbaum et al., 2012). There are potential negative trade-offs between
afforestation for climate mitigation purposes and other ecosystem
services, such as water supply (Jackson et al., 2005) and biodiversity
maintenance (CBD, 2012; Russell et al., 2012).
It has been suggested (Ridgwell et al., 2009) that planting large areas
of crop varieties with highly reflective leaves could help mitigate global
change. Model analyses indicate this “geo-engineering” strategy would
be marginally effective at high latitudes, but have undesirable climate
consequences at low latitudes. Measurements of leaf albedo in major
crops show that the current range of variability is insufficient to make
a meaningful difference to the global climate (Doughty et al., 2011).
4.4. Adaptation and Its Limits
4.4.1. Autonomous Adaptation
by Ecosystems and Wild Organisms
Autonomous adaptation (see Glossary under adaptation) refers to the
adjustments made by ecosystems, including their human components,
without external intervention, in response to a changing environment
(Smit et al., 2000)—also called “spontaneous adaptation” (Smit et al.,
2007). In the context of human systems it is sometimes called “coping
capacity.” The capacity for autonomous adaptation is part of resilience
but is not exactly synonymous (Walker et al., 2004).
A
ll social and ecological systems have some capacity for autonomous
adaptation. Ecosystems that have persisted for a long time can
reasonably be inferred to have a high capacity for autonomous
adaptation, at least with respect to the variability that they have
experienced in the past. An environmental change that is more rapid
than in the past or is accompanied by other stresses may exceed the
previously demonstrated adaptive capacity of the system. Adaptation
at one level, for instance by organisms in a community, can confer
greater resilience at higher organization levels, such as the ecosystem
(Morecroft et al., 2012). The mechanisms of autonomous adaptation of
organisms and ecosystems consist of changes in the physiology, behavior,
phenology, or physical form of organisms, within the range permitted
by their genes and the variety of genes in the population; changes in the
genetic composition of the populations; and change in the composition
of the community, through in- or out-migration or local extinction.
The ability to project impacts of climate change on ecosystems is
complicated by the potential for species to adapt. Adaptation by
individual species increases their ability to survive and flourish under
different climatic conditions, possibly leading to lower risks of extinction
than predicted from statistical correlations between current distribution
and climate (Botkin et al., 2007). It may also affect their interactions
with other species, leading to disruption of the biotic community (Visser
and Both, 2005).
4.4.1.1. Phenological
Changes in phenology are occurring in many species and locations
(Section 4.3.2.1). Further evidence since AR4 shows how this can be
an adaptation to climate change, but also the limits to phenological
adaptation. An organism’s phenology is typically highly adapted to the
climate seasonality of the environment in which it evolved. Species
unable to adjust their phenological behavior will be negatively affected,
particularly in highly seasonal habitats (Both et al., 2010).
Moreover, the phenology of any species also needs to be keyed to the
phenology of other species with which it interacts, such as competitors,
food species, and pollinators. Systematic cross-taxa studies indicate
different rates of phenological change for different species and trophic
levels (Parmesan, 2007; Cook et al., 2008; Thackeray et al., 2010). If
adaptation is insufficiently rapid or coordinated between interdependent
species, disruption of ecological features such as trophic cascades,
competitive hierarchies, and species coexistence is inferred to result
(Nakazawa and Doi, 2012). Lack of coordination can occur if one of the
species is cued to environmental signals that are not affected by climate
change, such as day length (Parmesan, 2006). Increasing temperatures
may bring species either more into or out of synchrony, depending on
their respective starting positions (Singer and Parmesan, 2010), although
evidence is more toward a loss of synchrony (Thackeray et al., 2010).
Changes in interspecific interactions, such as predator-prey or
interspecific competition for food, stemming from changes in phenological
characteristics and breakdown in synchrony between species have been
observed. For example, bird breeding is most effective when synchronized
with the availability of food, so changes in the phenology of food
supplies can exert a selective pressure on birds. In a study of 100

322
Chapter 4 Terrestrial and Inland Water Systems
4
E
uropean migratory bird species, those that advanced their arrival date
showed stable or increasing populations between 1990 and 2000, while
those that did not adjust their arrival date on average showed declining
populations (Møller et al., 2008). In a comparison of nine Dutch
populations of the migratory pied flycatcher (Ficedula hypoleuca) between
1987 and 2003, populations declined by 90% in areas where food
peaked early in the season and the arrival of the birds was mis-timed,
but not in areas with a later food peak that could still be exploited by
early breeding birds (Both et al., 2006). However, compensating
processes can exist: for example, in a 4-decade study of great tits (Parus
major), breeding populations were buffered against phenological
mismatch due to relaxed competition between individual fledglings
(Reed et al., 2013). Between 1970 and 1990, changes in migration date
did not predict changes in population sizes (Møller et al., 2008).
Bird breeding can also be affected by phenological shifts in competing
species and predators. Between 1953 and 2005 in southwestern
Finland, the onset of breeding of the resident great tit Parus major and
the migratory pied flycatcher (Ficedula hypoleuca) became closer to
each other, increasing competition between them (Ahola et al., 2007).
The edible dormouse (Glis glis), a nest predator, advanced its hibernation
termination by -8 days per decade in the Czech Republic between 1980
and 2005 due to increasing annual spring air temperatures, leading to
increased nest predation in three out of four surveyed bird species
(Adamik and Kral, 2008).
Plant-insect interactions have also been observed to change. In Illinois,
USA, the pattern of which plants were pollinated by which bees were
altered by differing rates of phenological shifts and landscape changes
over 120 years, with 50% of bee species becoming locally extinct (Burkle
et al., 2013). Increasing asynchrony of the winter moth (Operophtera
brumata) and its feeding host oak tree (Quercus robur) in the Netherlands
was linked to increasing spring temperatures but unchanging winter
temperatures (van Asch and Visser, 2007). Warmer temperatures shorten
the development period of European pine sawfly larvae (Neodiprion
sertifer), reducing the risk of predation and potentially increasing the
risk of insect outbreaks, but interactions with other factors including
day length and food quality may complicate this prediction (Kollberg et
al., 2013). In North America, the spruce budworm (Choristaneura
fumiferana) lays eggs with a wide range of emergence timings, so the
population as a whole is less sensitive to changing phenology of host
trees (Volney and Fleming, 2007).
The environmental cues for phenological events are complex and multi-
layered (Körner and Basler, 2010; Singer and Parmesan, 2010). For
instance, many late-succession temperate trees require a chilling period
in winter, followed by a threshold in day length, and only then are
sensitive to temperature. As a result, simple projections of current
phenological trends may be misleading, since the relative importance
of cues can change (Cook et al., 2012b). The effects are complex and
sometimes apparently counterintuitive, such as the increased sensitivity
of flowering in high-altitude perennial herbs in the Rocky Mountains
to frost because plants begin flowering earlier as a result of earlier
snowmelt (Inouye, 2008).
It has been suggested that shorter generation times give greater
opportunity for autonomous adaptation through natural selection
(
Rosenheim and Tabashnik, 1991; Bertaux et al., 2004), but a standardized
assessment of 25,532 rates of phenological change for 726 UK taxa
indicated that generation time had only limited influence on adaptation
rates (Thackeray et al., 2010).
There is high confidence (much evidence, medium agreement) that
climate change-induced phenological shifts will continue to alter the
interactions between species in regions with a marked seasonal cycle.
4.4.1.2. Evolutionary and Genetic
Since AR4 there has been substantial progress in defining the concepts
and tools necessary for documenting and predicting evolutionary and
genetic responses to recent and future climate change, often referred
to as “rapid evolution.” Evolution can occur through many mechanisms,
including selection of existing genes or genotypes within populations,
hybridization, mutation, and selection of new adaptive genes and perhaps
even through epigenetics (Chevin et al., 2010; Chown et al., 2010;
Lavergne et al., 2010; Paun et al., 2010; Hoffmann and Sgro, 2011;
Anderson et al., 2012a; Donnelly et al., 2012; Franks and Hoffmann, 2012;
Hegarty, 2012; Merilä, 2012; Bell, 2013; Zhang et al., 2013). Mechanisms
such as selection of existing genes and genotypes, hybridization, and
epigenetics can lead to adaptation in very few generations, while others,
notably mutation and selection of new genes, typically take many tens of
generations. This means that species with very fast life cycles, for example,
bacteria, should in general have greater capacity to respond to climate
change than species with long life cycles, such as large mammals and trees.
There is a paucity of observational or experimental data that can be used
for detection and attribution of recent climate effects on evolution.
4.4.1.2.1. Observed evolutionary and genetic responses
to rapid changes in climate
There is a small but growing body of observations supporting the AR4
assessment that some species may have adapted to recent climate
warming or to climatic extremes through genetic responses (e.g.,
plants: Franks and Weis, 2008; Hill et al., 2011; Anderson et al., 2012b;
vertebrates: Ozgul et al., 2010; Phillimore et al., 2010; Husby et al., 2011;
Karell et al., 2011; insects: Buckley et al., 2012; van Asch et al., 2012).
Karell et al. (2011) found increasing numbers of brown genotypes of
the tawny owl (Strix aluco) in Finland over the course of the last 28
years and attributed it to fewer snow-rich winters, which creates strong
selection pressure against the white genotype. Earlier spawning by the
common frog (Rana temporaria) in Britain could be attributed largely
to local genetic adaptation to increasing spring temperatures (Phillimore
et al., 2010). Using a combination of models and observations, Husby
et al. (2011) have built a case for detection and attribution of genetic
adaptation in an insectivorous bird and in an herbivorous insect that has
tracked warming-related changes in the budburst timing of its host tree
(van Asch et al., 2012). In contrast, many species appear to be maladapted
to changing climates, in part because factors such as limited existing
genetic variation, weak heritability of adaptive traits, or conflicting
constraints on adaptation create low potential for rapid evolution
(Knudsen et al., 2011; Ketola et al., 2012; Merilä, 2012; Mihoub et al.,
2012). Most studies of rapid evolution suffer from methodological

323
Terrestrial and Inland Water Systems Chapter 4
4
w
eaknesses, making it difficult to demonstrate clearly a genetic basis
underlying observed phenotypic responses to environmental change
(Gienapp et al., 2008; Franks and Hoffmann, 2012; Hansen et al., 2012;
Merilä, 2012). Rapid advances in quantitative genetics, genomics, and
phylogenetics, combined with recent progress on conceptual frameworks,
will substantially improve the detection and attribution of genetic
responses to changing climate over the next few years (Davis, C.C. et
al., 2010; Salamin et al., 2010; Hoffmann and Sgro, 2011). In sum, there
are few observational studies of rapid evolution and difficulties in
detection and attribution, so there is only medium confidence that some
species have responded to recent changes in climate through genetic
adaptations, and insufficient evidence to determine if this is a widespread
phenomenon (thus low confidence for detection and attribution across
all species; Figure 4-4).
The ability of species to adapt to new environmental conditions through
rapid evolutionary processes can also be inferred from the degree to
which environmental niches are conserved when environment is
changed. There is evidence that environmental niches are conserved for
some species under some conditions (plants: Petitpierre et al., 2012;
birds: Monahan and Tingley, 2012; review: Peterson et al., 2011), but
also evidence suggesting that environmental niches can evolve over time
scales of several decades following changes in climate (Broennimann
et al., 2007; Angetter et al., 2011; Konarzewski et al., 2012; Leal and
Gunderson, 2012; Lavergne et al., 2013). The paleontological record
provides insight into evolutionary responses in the face of natural climate
variation. In general, environmental niches appear to be broadly
conserved through time although there are insufficient data to determine
the extent to which genetic adaptation has attenuated range shifts and
changes in population size (Peterson et al., 2011; Willis and MacDonald,
2011). Phylogeographic reconstructions of past species distributions
suggest that hybridization may have helped avoid extinctions during
cycles of glaciation and could also play a key role in future adaptation
(Hegarty, 2012; Soliani et al., 2012). There is new evidence that epigenetic
mechanisms, such as DNA methylation, could allow very rapid adaptation
to climate (Paun et al., 2010; Zhang et al., 2013).
4.4.1.2.2. Mechanisms mediating rapid evolutionary response
to future climate change
Studies of genetic variability across species ranges, and models that
couple gene flow with spatially explicit population dynamics, suggest
counterintuitive responses to climate change. Too much or too little gene
flow to populations at range margins can create fragile, maladapted
populations, which is in contrast to the current wisdom that populations
at the range margins may be best adapted to global warming (Bridle et
al., 2010; Hill et al., 2011). Conversely, there is evidence from experiments,
models, and observations that populations in the center of species
ranges may in some cases be more sensitive to environmental change
than those at range boundaries (Bell and Gonzalez, 2009). Generalization
is complicated by the interactions between local adaptation, gene flow,
population dynamics, and species interactions (Bridle et al., 2010;
Norberg et al., 2012).
Substantial progress has been made since AR4 in developing models
for exploring whether genetic adaptation is fast enough to track climate
c
hange. Models of long-lived tree species suggest that existing genetic
variation may be sufficient to slightly attenuate negative impacts of
future climate change (Kuparinen et al., 2010; Kremer et al., 2012).
However, these studies also indicate that adaptive responses will lag
far behind even modest rates of projected climate change, owing to the
very long generation time of trees. In a species with much shorter
generation times, the great tit (Parus major), Gienapp et al. (2013) found
that modeled avian breeding times tracked climate change, only at low
to moderate rates of change. For a herbivorous insect with an even
faster life cycle, van Asch et al. (2007, 2012) predicted that rapid
evolution of the phenological response should have allowed it to track
recent warming, which it has.
More broadly, models suggest that species with short generation times
(1 year or less) potentially have the capacity to genetically adapt to
even the most rapid rates of projected climate change given large
enough present-day populations, but species with longer generation
times or small populations could be at risk of extinction at moderate to
high rates of climate change (Walters et al., 2012; Vedder et al., 2013).
Recent experimental and theoretical work on “evolutionary rescue”
shows that long-term avoidance of extinction through genetic adaptation
to hostile environments is possible, but requires large initial genetic
variation and population sizes and is accompanied by substantial loss
of genetic diversity, reductions in population size, and range contractions
over many generations before population recovery (Bell, 2013; Schiffers
et al., 2013).
Model-based projections must be viewed with considerable caution
because there are many evolutionary and ecological mechanisms not
accounted for in most models that can either speed up or inhibit
heritable adaptation to climate change (Cobben et al., 2012; Norberg et
al., 2012; Kovach-Orr and Fussmann, 2013). In some cases, accounting
for evolutionary processes in models even leads to predictions of greater
maladaptation to climate change, resulting in rapid population declines
(Hendry and Gonzalez, 2008; Ferriere and Legendre, 2013). Phenotypic
plasticity is thought to generally improve the odds of adaptation to
climate change. High plasticity in the face of climate change that has
low fitness costs can greatly improve the odds of adaptation; however,
plasticity with high costs leads to only modest amounts of adaptation
(Chevin et al., 2010).
AR4 concluded that “projected rates of climate change are very likely
to exceed rates of evolutionary adaptation in many species (high
confidence)” (Fischlin et al., 2007). Work since then provides a similar, but
more nuanced view of rapid evolution in the face of future climate change.
The lack of adaptation in some species to recent changes in climate, broad
support for niche conservatism, and models showing limited adaptive
capacity in species with long generation times all indicate that high
rates of climate change (RCP8.5) will exceed the adaptive capacities of
many species (high confidence). On the other hand, evidence from
observations and models also indicates that there is substantial capacity
for genetic adaptation to attenuate phenological shifts, population
declines, and local extinctions in many species, especially for low rates
of climate change (RCP2.6) (high confidence). Projected adaptation to
climate change is frequently characterized by population declines and
loss of genetic diversity for many generations (medium confidence),
thereby increasing species vulnerability to other pressures.

324
Chapter 4 Terrestrial and Inland Water Systems
4
4.4.1.3. Migration of Species
This mode of adaptation has been extensively dealt with in Section 4.3.2.5.
It is anticipated that the observed movement of species—individually
and collectively—will continue in response to shifting climate patterns.
Its effectiveness as an adaptation mechanism is constrained by three
factors. First, the rate of migration for many species, in many regions of
the world, is slower than the rate of movement of the climate envelope
(see Figure 4-5). Second, the ecosystem interactions can remain intact
only if all parts of the ecosystem migrate simultaneously and at the same
rate. Third, the contemporary landscape and inland water systems contain
many barriers to migration, in the form of habitat fragmentation, roads,
human settlements, and dams. Mountain ecosystems are less constrained
by these factors than flat-land ecosystems, but have additional
impediments for species already close to the top of the mountain.
4.4.2. Human-Assisted Adaptation
Human-assisted adaptation means a deliberate intervention with the
intent of increasing the capacity of the target organism, ecosystem, or
socio-ecological system to survive and function at an acceptable level in
the presence of climate change. It is also known as “planned adaptation”
(Smit et al., 2007). This chapter focuses less on the adaptation of people,
human communities, and infrastructure, as they are the topics of
Chapters 8 to 17, and more on non-human organisms and ecosystems,
while acknowledging the importance of the human elements within
the ecosystem. Intervention in this context means a range of actions,
including ensuring the presence of suitable habitat and dispersal pathways;
reducing non-climate stressors; and physically moving organisms and
storing and establishing them in new places. In addition to the other
approaches assessed in this section, “Ecosystem-Based Adaptation” (see
Box CC-EA) provides an option that integrates the use of biodiversity
and ecosystem services into climate change adaptation strategies in
ways that can optimize co-benefits for local communities and carbon
management, as well as reduce the risks associated with possible
maladaptation. Note that there are risks associated with all forms of
human-assisted adaptation (see Section 4.4.4), particularly in the presence
of far-from-perfect predictive capabilities (Willis and Bhagwat, 2009).
4.4.2.1. Reduction of Non-Climate Stresses and Restoration of
Degraded Ecosystems
The alleviation of other stresses acting on ecosystems is suggested to
increase the capacity of ecosystems to survive, and adapt to, climate
change, as the effects are generally either additive or compounding.
Ecosystem restoration is one way of alleviating such stresses while
increasing the area available for adaptation (Harris et al., 2006). Building
the resilience of at-risk ecosystems by identifying the full set of drivers
of change and most important areas and resources for protection is the
core of the adaptation strategy for the Arctic (Christie and Sommerkorn,
2012). Protective and restorative actions aimed at increasing resilience can
also be a cost-effective means as part of an overall adaptation strategy to
help people to adapt to the adverse effects of climate change and may
have other social, economic, and cultural benefits. This is part of
“ecosystem-based adaptation” (Colls et al., 2009; Box CC-EA).
4.4.2.2. The Size, Location, and Layout of Protected Areas
Additions to, or reconfigurations of, the protected area estate are
commonly suggested as pre-adaptations to projected climate changes
(Heller and Zavaleta, 2009). This is because for most protected areas,
under plausible scenarios of climate change, a significant fraction of
the biota will no longer have a viable population within the present
protected area footprint. It is noted that the extant geography of
protected areas is far from optimal for biodiversity protection even
under the current climate; that most biodiversity exists outside rather
than in protected areas and this between-protected area matrix is as
important; that it is usually cheaper to acquire land proactively in the
areas of projected future bioclimatic suitability than to correct the current
non-optimality and then later add on areas to deal with climate change
as it unfolds (Hannah et al., 2007); and that the existing protected area
network will still have utility in future climates, even though it may
contain different species (Thomas et al., 2012).
Hickler et al. (2012) analyzed the layout of protected areas in Europe
and concluded that under projected 21st century climate change a third
to a half of them would potentially be occupied by different vegetation
than they currently represent. The new areas that need to be added to
the existing protected area network to ensure future representativeness
is situation specific, but some general design rules apply: orientation
along climate gradients (e.g., altitudinal gradients) is more effective
than orientation across them (Roux et al., 2008); regional scale planning
is more effective than treating each local case independently because
it is the network of habitats and protected areas that confers resilience
rather than any single element (Heller and Zavaleta, 2009); and better
integration of protected areas with a biodiversity-hospitable landscape
outside is more effective than treating the protected areas as islands
(Willis and Bhagwat, 2009). Dunlop et al. (2012) assessed the implications
of climate change for biodiversity conservation in Australia and found
many opportunities to facilitate the natural adaptation of biodiversity,
including expanding the network of protected areas and restoring habitat
at a large scale.
4.4.2.3. Landscape and Watershed Management
The need to include climate change into the management of vulnerable
ecosystems is explicitly included in the strategic goals of the Convention
on Biological Diversity. Oliver et al. (2012b) developed decision trees
based on three scenarios: (1) adversely sensitive, where areas within the
species current geographical range will become climatically unsuitable
with a changing climate; (2) climate overlap, where there are areas that
should remain climatically suitable within the species’ range; and (3)
new climatic space, which refers to areas outside of the current range
that are projected to become suitable. Heller and Zavaleta (2009)
reviewed recommendations in the published literature and argue that
the majority of them, such as increase habitat heterogeneity of sites
and connectivity of habitats across landscapes, lack sufficient specificity
to ensure the persistence of many species and related ecosystem
services to ongoing climate change. To date, recommendations are
overwhelmingly focused on ecological data, neglecting social science
insights. Few resources or capacity exist to guide adaptation planning
processes at any scale.

325
Terrestrial and Inland Water Systems Chapter 4
4
Climate-induced impacts to hydrological and thermal regimes in
freshwater systems can be offset through improved management of
environmental flow releases from reservoirs (Arthington et al., 2006,
2010 and references therein; Poff et al., 2010). Protection and restoration
of riparian vegetation in small stream systems provide an effective strategy
to moderate temperature regimes and offset warming, and protect
water quality for downstream ecosystems and water supply areas
(Davies, 2010; Capon et al., 2013).
General principles for management adaptations were summarized from
a major literature review by West et al. (2009). They suggest that in the
context of climate change, successful management of natural resources
will require cycling between “managing for resilience” and “managing
for change.” This requires the anticipation of changes that can alter the
impacts of grazing, fire, logging, harvesting, recreation, and so on. At
the national level, principles to facilitate adaptation include (1)
management at appropriate scales, and not necessarily the scales of
convenience or tradition; (2) increased collaboration among agencies;
(3) rational approaches for establishing priorities and applying triage;
and (4) management with the expectation of ecosystem change, rather
than keeping them as they have been. Barriers and opportunities were
divided into four categories: (1) legislation and regulations, (2)
management policies and procedures, (3) human and financial capital,
and (4) information and science.
Steenberg et al. (2011) simulated the effect on adaptive capacity of
three variables related to timber harvesting: the canopy-opening size
of harvests, the age of harvested trees within a stand, and the species
composition of harvested trees within a stand. The combination of all
three adaptation treatments allowed target species and old forest to
remain reasonably well represented without diminishing the timber
supply. This minimized the trade-offs between management values and
climate adaptation objectives. Manipulation of vegetation composition
and stand structure has been proposed as a strategy for offsetting
climatic change impacts on wildfires in Canada. Large areas of boreal
forests are currently being harvested and there may be opportunities
for using planned manipulation of vegetation for management of future
wildfire risks. This management option could also provide an additional
benefit to the use of assisted species migration because the latter would
require introducing non-flammable broadleaves species into forests that
are otherwise highly flammable (Girardin et al., 2013b; Terrier et al.,
2013). Harvesting practices, such as partial cuts that limit the opening
of the forest cover created by harvest, will be a key element to maintain
diverse forest compositions and age class distributions in boreal forests.
Another sound option for decreasing the exposure of silvicultural
investments to an increasing fire danger is to use tree species requiring
a shorter rotation (Girardin et al., 2013a).
4.4.2.4. Assisted Migration
Assisted migration has been proposed when fragmentation of habitats
limits migration potential or when natural migration rates are outstripped
by the pace of climate change (Hoegh-Guldberg et al., 2008; Vitt et al.,
2010; Chmura et al., 2011; Loss et al., 2011; Ste-Marie et al., 2011). The
options for management can be summarized as: (1) try to maintain or
improve existing habitat or environment so that species do not have to
move (e.g., Settele and Kühn, 2009); (2) maintain or improve migration
corridors, including active management to improve survival along the
moving margin of the distribution (Lawson et al., 2012); and (3) directly
translocate species or genetically distinct populations within a species
(Aitken et al., 2008; Hoegh-Guldberg et al., 2008; Rehfeldt and Jaquish,
2010; Loss et al., 2011; Pedlar et al., 2012). There is low agreement
whether it is better to increase the resilience to climate change of
ecosystems as they currently occur, or to enhance capacity of ecosystems
to transform in the face of climate change (Richardson et al., 2009).
There is high agreement that maintaining or improving migration
corridors or ecological networks is a low-regret strategy, partly because
it is also seen as useful in combatting the negative effects of habitat
fragmentation on population dynamics (Hole et al., 2011; Jongman et
al., 2011). This approach has the benefit of improving the migration
potential for large numbers of species and is therefore a more ecosystem-
wide approach than assisted migration for individual species. However,
observational and modeling studies show that increases in habitat
connectivity do not always improve the population dynamics of target
Frequently Asked Questions
FAQ 4.6 | Can ecosystems be managed to help them and people to adapt to climate change?
The ability of human societies to adapt to climate change will depend, in large measure, on the management of
terrestrial and inland freshwater ecosystems. A fifth of global human-caused carbon emissions today are absorbed
by terrestrial ecosystems; this important carbon sink operates largely without human intervention, but could be
increased through a concerted effort to reduce forest loss and to restore damaged ecosystems, which also co-benefits
the conservation of biodiversity.
The clearing and degradation of forests and peatlands represents a source of carbon emissions to the atmosphere
which can be reduced through management; for instance, there has been a three-quarters decline in the rate of
deforestation in the Brazilian Amazon in the last 2 decades. Adaptation is also helped through more proactive
detection and management of wildfire and pest outbreaks, reduced drainage of peatlands, the creation of species
migration corridors, and assisted migration.

326
Chapter 4 Terrestrial and Inland Water Systems
4
s
pecies, may decrease species diversity, and may also facilitate the spread
of invasive species (Cadotte, 2006; Brisson et al., 2010; Matthiessen et
al., 2010).
There is medium agreement that the practice of assisted migration of
targeted species is a useful adaptation option (Hoegh-Guldberg et al.,
2008; Vitt et al., 2009; Willis and Bhagwat, 2009; Loss et al., 2011;
Hewitt et al., 2011). The velocity of 21st century climate change and
substantial habitat fragmentation in large parts of the world means
that many species will be unable to migrate or adapt fast enough to
keep pace with climate change (Figure 4-5), posing problems for long-
term survival of the species. Some ecologists believe that careful selection
of species to be moved would minimize the risk of undesirable impacts on
existing communities or ecosystem function (Minteer and Collins, 2010),
but others argue that the history of intentional species introductions
shows that the outcomes are unpredictable and in many cases have
had disastrous impacts (Ricciardi and Simberloff, 2009). The number of
species that require assisted migration could easily overwhelm funding
capacity (Minteer and Collins, 2010). Decisions regarding which species
should be translocated are complex and debatable, given variability
among and within species and the ethical issues involved (Aubin et al.,
2011; Winder, R. et al., 2011).
4.4.2.5. Ex Situ Conservation
Conservation of plant and animal genetic resources outside of their
natural environment—in gardens, zoos, breeding programs, seed
banks, or gene banks—has been widely advocated as an “insurance”
against both climate change and other sources of biodiversity loss and
impoverishment (Khoury et al., 2010). There are many examples of
existing efforts of this type, some with global scope (e.g., Millennium
Seed Bank, Svalbard Vault, Frozen Ark, Global Genome Initiative, and
others; Lermen et al., 2009; Rawson et al., 2011). Knowledge of which
genetic variants within a species have more potential for adaptation to
climate change could help prioritize the material stored (Michalski et
al., 2010).
Several issues remain largely unresolved (Li and Pritchard, 2009). The
physiological, institutional, and economic sustainability of such efforts
into the indefinite future is unclear. The fraction of the intraspecific
variation that needs to be preserved for future viability and how much
g
enetic bias is introduced by collecting relatively small samples
from restricted locations, and then later by the selection pressures
inadvertently applied during ex situ maintenance are unknown. Despite
some documented successes, it remains uncertain whether it is always
possible to reintroduce species successfully into the wild after generations
of ex situ conservation.
4.4.3. Consequences and Costs of Inaction
and Benefits of Action
Failure to reduce the magnitude or rate of climate change will plausibly
lead to changes (often decreases) in the value of ecosystem services
provided, or incur costs in order to maintain or restore the services or adapt
to their decline. There are several sources of such costs: administration
and assessment, implementation, and opportunity costs, including
financial cost. Owing to the number of assumptions made, knowledge
gaps, and recognized uncertainties, such result should be employed with
caution. A systematic review of costs related to ecosystems and climate
change by Rodriguez-Labajos (2013) shows that the monetary and non-
monetary costs are distributed across all ecosystem service categories.
It also discusses the potential and limits of monetary cost calculations,
and issues of timing, trade-offs, and the unequal distribution of costs.
A comprehensive monetary estimate of the effects of climate change on
ecosystem service provision is not available. The Millennium Ecosystem
Assessment (2005c,d,e) included climate change among the direct
drivers of ecosystems change and devoted a chapter to the necessary
responses. Building on results of the IPCC, the Millennium Ecosystem
Assessment offered some estimated costs of action: complying with the
Kyoto protocol for industrial countries would range between 0.2 and 2%
of GDP; a modest stabilization target of 450 ppm CO
2
in the atmosphere
over the 21st century would range from 0.02 to 0.1% of global-average
GDP per year. TEEB (2009) underlined priorities in the ecosystem service-
climate change coupling (reduction targets in relation to coral reefs,
forest carbon markets and accounting, and ecosystem investment for
mitigation), without going in depth into analysis of the cost types
involved. The Cost of Policy Inaction (COPI) Project (ten Brink et al., 2008)
estimated the monetary costs of not meeting the 2010 biodiversity goals.
Their model incorporates climate change, among other pressures,
through an impaired quality of land, in terms of species abundance in
diverse land use categories. They conclude that the cumulative losses
Frequently Asked Questions
FAQ 4.7 | What are the economic costs of changes in ecosystems due to climate change?
Climate change will certainly alter the services provided by most ecosystems, and for high degrees of change, the
overall impacts are most likely to be negative. In standard economics, the value of services provided by ecosystems
are known as externalities, which are usually outside the market price system, difficult to evaluate, and often ignored.
A good example is the pollination of plants by bees and birds and other species, a service that may be negatively
affected by climate change. Pollination is critical for the food supply as well as for overall environmental health. Its
value has been estimated globally at US$350 billion for the year 2010 (range of estimates of US$200 to 500 billion).

327
Terrestrial and Inland Water Systems Chapter 4
4
o
f welfare due to land use changes, in terms of loss of ecosystem
services, could reach an annual amount of EUR 14 trillion (based on
2007 values) in 2050, which may be equivalent to 7% of projected
global GDP for that year. Eliasch (2008) estimates the damage costs to
forests as reaching US$1 trillion a year by 2100. The study used the
probabilistic model employed by Stern (2006), which did not value
effects on biodiversity or water-related ecosystem services.
The studies to date agree on the following points. First, climate change
has already caused a reduction in ecosystem services that will become
more severe as climate change continues. Second, ecosystem-based
strategies to mitigate climate change are cost effective, although more
difficult to implement (i.e., more costly) in intensively managed ecosystems
such as farming lands. Third, accurately estimating the monetary costs
of reduction in ecosystem services that are not marketed is difficult. The
provision of monetized costs tends to sideline the non-monetized
political, social, and environmental costs relevant for decision making.
Finally, there is a large funding gap between the cost of actions necessary
to protect ecosystem services against climate change and the actual
resources available.
In addition to direct costs, further costs may result from trade-offs
between services: for example, afforestation for climate mitigation and
urban greening for climate adaptation may be costly in terms of water
provision (Chisholm, 2010; Jenerette et al., 2011; Pataki et al., 2011).
Traditional agriculture preserves soil carbon sinks, supports on-site
biodiversity, and uses less fossil fuel than high-input agriculture
(Martinez-Alier, 2011) but, due to the typically lower per hectare yields,
may require a larger area to be dedicated to cropland. Leaving aside
the contested (Searchinger et al., 2008; Plevin et al., 2010) effectiveness
of biofuels as a mitigation strategy, there is evidence of their disruptive
effect on food security, land tenure, labor rights, and biodiversity in
several parts of the world (Obersteiner et al., 2010; Tirado et al., 2010).
4.4.4. Unintended Consequences
of Adaptation and Mitigation
Actions taken within the terrestrial and freshwater system domain or in
other sectors to mitigate or adapt to climate change can have unintended
consequences. Some issues relevant to this section are also found in
Section 14.7 and the Working Group III contribution to the AR5.
Several of the alternatives to fossil fuel require extensive use of the land
surface and thus have a direct impact on terrestrial ecosystems and an
indirect impact on inland water systems (Paterson et al., 2008; Turner et
al., 2010). As an illustration, the RPC2.6 scenario involves both bioenergy
and renewables as major components of the energy mix (Box 4-1; van
Vuuren et al., 2011).
Policy shifts in developed countries favor the expansion of large-scale
bioenergy production, which places new pressures on terrestrial and
freshwater ecosystems (Searchinger et al., 2008; Lapola et al., 2010),
either through direct use of land or water or indirectly by displacing
food crops, which must then be grown elsewhere. Over the past decade
there has been a global trend to reduced rates of forest loss; it is unclear
if this will continue in the face of simultaneously rising food and biofuel
d
emand (Wise et al., 2009; Meyfroidt and Lambin, 2011). The EU
Renewable Energy Sources Directive is estimated to have only a
moderate influence on European forests provided that the price paid
by the bioenergy producers remained below US$50 to 60 per cubic
meter of wood (Moiseyev et al., 2011). However, a doubled growth rate
for bioenergy until 2030 would have major consequences for the global
forest sector, including a reduction of forest stocks in Asia of 2 to 4%
(Buongiorno et al. 2011). By 2100 in RCP2.6, bioenergy crops are
projected to occupy approximately 4 million km
2
, about 7% of global
cultivated land projected at the time. Modification of the landscape and
the fragmentation of habitats are major influences on extinction risks
(Fischer and Lindenmayer, 2007), especially if native vegetation cover is
reduced or degraded, human land use is intensive, and “natural” areas
become disconnected. Hence, additional extensification of cultivated
areas for energy crops may contribute to extinction risks. Some bioenergy
crops may be invasive species (Raghu et al., 2006).
Abandoned former agricultural land could be used for biomass production
(McAlpine et al., 2009). However, such habitats may be core elements
in cultural landscapes of high conservation value, with European
species-rich grasslands often developed from abandoned croplands
(Hejcman et al., 2013).
Damming of river systems for hydropower can cause fragmentation of the
inland water habitat with implications for fish species, and monitoring
studies indicate that flooding of ecosystems behind the dams can lead
to declining populations, for example, of amphibians (Brandão and
Araújo, 2007). Reservoirs can be a sink of CO
2
but also a source of
biogenic CO
2
and CH
4
; this issue is discussed in WG III AR5 Section 7.8.1.
Wind turbines can kill birds and bats (e.g., Barclay et al., 2007), and
inappropriately sited wind farms can negatively impact on bird
populations (Drewitt and Langston, 2006). Effects can be reduced by
careful siting of turbines, for example by avoiding migration routes
(Drewitt and Langston, 2006). Estimating mortality rates is complex and
difficult (Smallwood, 2007) but techniques are being developed to
inform siting decisions and impact assessments (Péron et al., 2013).
Wind farms in Europe and the USA are estimated to cause between 0.3
and 0.4 wildlife fatalities per gigawatt-hour of electricity, compared to
approximately 5.2 wildlife fatalities per gigawatt-hour for nuclear and
fossil-fuel power stations (Sovacool, 2009; but see Willis, C.K.R. et al.,
2010). One study found on-site bird populations to be generally affected
more by windfarm construction than subsequent operation, with some
populations recovering after construction (Pearce-Higgins et al., 2012).
Large-scale solar farms could impact local biodiversity if poorly sited,
but the impact can be reduced with appropriate planning (Tsoutsos et
al., 2005). Solar photovoltaic installations can decrease local surface
albedo, giving a small positive radiative forcing. There are some plausible
local circumstances in which this may be a consideration, but in general
the climate effect is estimated to be 30 times smaller than the avoided
radiative forcing arising from substituting fossil fuels with PV (Nemet,
2009).
Relocation or expansion of agricultural areas and settlements as climate
change adaptation measures could pose risks of habitat fragmentation
and loss similar to those discussed above in the context of mitigation

328
Chapter 4 Terrestrial and Inland Water Systems
4
t
hrough bio-energy. Assisted migration (see Section 4.4.2.4) may
directly conflict with other conservation priorities, for example by
facilitating the introduction of invasive species (Maclachlan et al., 2007).
4.5. Emerging Issues and Key Uncertainties
Detecting the presence and location of thresholds in ecosystem response
to climate change, specifically the type of thresholds characterized as
tipping points, remains a major source of uncertainty with high potential
consequences. In general (Field et al., 2007), negative feedbacks
currently dominate the climate-ecosystem interaction. For most ecological
processes, increasing magnitude of warming shifts the balance toward
positive rather than negative feedbacks (Field et al., 2007). In several
regions, such as the boreal ecosystems, positive feedbacks may become
dominant, under moderate warming. For positive feedbacks to propagate
into “runaway” processes leading to a new ecosystem state, the
strength of the feedback has to exceed that of the initial perturbation.
This has not as yet been demonstrated for any large-scale, plausible,
and immanent ecological process, but the risk is non-negligible and the
consequences if it did occur would be severe; thus further research is
needed.
The issue of biophysical interactions between ecosystem state and the
climate, over and above the effects mediated through GHGs, is emerging
as significant in many areas. Such effects include those caused by
changes in surface reflectivity (albedo) or the partitioning of energy
between latent energy and sensible heat.
Uncertainty in predicting the response of terrestrial and freshwater
ecosystems to climate and other perturbations, particularly at the local
scale, remains a major impediment to determining prudent levels of
permissible change. A significant source of this uncertainty stems from
the inherent complexity of ecosystems, especially where they are coupled
to equally complex social systems. The high number of interactions can
lead to cascading effects (Biggs et al., 2011). Some of this uncertainty
can be reduced by better systems understanding, but some will remain
irreducible because of the failure of predictive models when faced with
certain types of complexity (such as those which lead to mathematical
bifurcations, a problem that is well known in climate science). Probabilistic
statements about the range of outcomes are possible in this context,
but ecosystem science is as yet mostly unable to conduct such analyses
routinely and rigorously. One consequence is the ongoing difficulty in
attributing observed changes unequivocally to climate change. More
comprehensive monitoring is a key element of the solution.
The consequences for species interactions of differing phenological or
movement-based responses to climate change are insufficiently known
and may make projections based on individual species models unreliable.
Studies of the combined effects of multiple simultaneous elements of
global change, such as the effects of elevated CO
2
and rising tropospheric
ozone on plant productivity—which have critical consequences for the
future sink strength of the biosphere, as they are of similar magnitude
but opposite sign—are needed as a supplement to the single-factor
experiments. For example, uncertainty on the magnitude of CO
2
fertilization is key for forest responses to climate change, particularly in
t
ropical forests, woodlands, and savannas (Cox et al., 2013; Huntingford
et al., 2013).
The effects of changes in the frequency or intensity of climate-related
extreme events, such as floods, cyclones, heat waves, and exceptionally
large fires on ecosystem change are probably equal to or greater than
shifts in the mean values of climate variables. These effects are
insufficiently studied and, in particular, are seldom adequately represented
in ESMs.
Understanding of the rate of climate change that can be tracked or
adapted to by organisms is as important as understanding the
magnitude of change they can tolerate. Despite being explicitly required
under Article 2 of the UNFCCC, rate studies are currently less developed
and more uncertain than magnitude (equilibrium) studies. This includes
evidence for the achievable migration rates of a range of species as
well as the rate of micro-evolutionary change.
The capacity for, and limits to, ecological and evolutionary adaptive
processes are known only in a few cases. The development and testing
of human-assisted adaptation strategies for their cost-effectiveness in
reducing risk are prerequisites for their widespread adoption.
The costs of the loss of biodiversity and ecosystem services as a result
of climate change are known for only a few cases, or are associated
with large uncertainties, as are the costs and benefits of assisting
ecosystems and species to adapt to climate change.
References
Aakala, T., T. Kuuluvainen, T. Wallenius, and H. Kauhanen, 2011: Tree mortality
episodes in the intact Picea abies-dominated taiga in the Arkhangelsk region
of northern European Russia. Journal of Vegetation Science, 22(2), 322-333.
Abatzoglou, J.T. and C.A. Kolden, 2011: Climate change in western US deserts:
potential for increased wildfire and invasive annual grasses. Rangeland Ecology
& Management, 64(5), 471-478.
Adamik, P. and M. Kral, 2008: Climate- and resource-driven long-term changes in
dormice populations negatively affect hole-nesting songbirds. Journal of
Zoology, 275(3), 209-215.
Adamik, P. and J. Pietruszkova, 2008: Advances in spring but variable autumnal trends
in timing of inland wader migration. Acta Ornithologica, 43(2), 119-128.
Adams, H.D., C.H. Luce, D.D. Breshears, C.D. Allen, M. Weiler, V.C. Hale, A.M.S. Smith,
and T.E. Huxman, 2012: Ecohydrological consequences of drought- and
infestation-triggered tree die-off: insights and hypotheses. Ecohydrology, 5(2),
145-159.
Adrian, R., C.M. O’Reilly, H. Zagarese, S.B. Baines, D.O. Hessen, W. Keller, D.M.
Livingstone, R. Sommaruga, D. Straile, E. Van Donk, G.A. Weyhenmeyer, and M.
Winder, 2009: Lakes as sentinels of climate change. Limnology and
Oceanography, 54(6), 2283-2297.
Ahl, D.E., S.T. Gower, S.N. Burrows, N.V. Shabanov, R.B. Myneni, and Y. Knyazikhin,
2006: Monitoring spring canopy phenology of a deciduous broadleaf forest
using MODIS. Remote Sensing of Environment, 104(1), 88-95.
Ahola, M.P., T. Laaksonen, T. Eeva, and E. Lehikoinen, 2007: Climate change can alter
competitive relationships between resident and migratory birds. Journal of
Animal Ecology, 76(6), 1045-1052.
Aiello-Lammens, M.E., M.L. Chu-Agor, M. Convertino, R.A. Fischer, I. Linkov, and
H.R. Akcakaya, 2011: The impact of sea-level rise on Snowy Plovers in Florida:
integrating geomorphological, habitat, and metapopulation models. Global
Change Biology, 17(12), 3644-3654.
Ainsworth, E.A., C.R. Yendrek, S. Sitch, W.J. Collins, and L.D. Emberson, 2012: The
effects of tropospheric ozone on net primary productivity and implications for
climate change. Annual Review of Plant Biology, 63(1), 637-661.

329
Terrestrial and Inland Water Systems Chapter 4
4
Aitken, S.N., S. Yeaman, J.A. Holliday, T.L. Wang, and S. Curtis-McLane, 2008:
Adaptation, migration or extirpation: climate change outcomes for tree
populations. Evolutionary Applications, 1(1), 95-111.
Alahuhta, J., J. Heino, and M. Luoto, 2011: Climate change and the future distributions
of aquatic macrophytes across boreal catchments. Journal of Biogeography,
38(2), 383-393.
Albert, K.R., J. Kongstad, I.K. Schmidt, H. Ro-Poulsen, T.N. Mikkelsen, A. Michelsen,
L. van der Linden, and C. Beier, 2012: Temperate heath plant response to dry
conditions depends on growth strategy and less on physiology. Acta Oecologica-
International Journal of Ecology, 45, 79-87.
Albert, K.R., H. Ro-Poulsen, T.N. Mikkelsen, A. Michelsen, L. Van der Linden, and C.
Beier, 2011: Effects of elevated CO
2
, warming and drought episodes on plant
carbon uptake in a temperate heath ecosystem are controlled by soil water
status. Plant Cell and Environment, 34(7), 1207-1222.
Aldous, A., J. Fitzsimons, B. Richter, and L. Bach, 2011: Droughts, floods and freshwater
ecosystems: evaluating climate change impacts and developing adaptation
strategies. Marine and Freshwater Research, 62(3), 223-231.
Alencar, A., D.C. Nepstad, and M.d.C. Vera Diaz, 2006: Forest understory fire in the
Brazilian Amazon in ENSO and non-ENSO years: area burned and committed
carbon emissions. Earth Interactions, 10, 6, 1-17, doi:10.1175/EI150.1.
Alencar, A., G.P. Asner, D. Knapp, and D. Zarin, 2011: Temporal variability of forest
fires in eastern Amazonia. Ecological Applications, 21(7), 2397-2412.
Alexander, H.D., M.C. Mack, S. Goetz, M. Loranty, P.S.A. Beck, K. Earl, S. Zimov, S.
Davydov, and C.C. Thompson, 2012: Carbon accumulation patterns during post-
fire succession in cajander larch (Larix cajanderi) forests of Siberia. Ecosystems,
15, 1065-1082.
Ali, A.A., O. Blarquez, M.P. Girardin, C. Hely, F. Tinquaut, A. El Guellab, V. Valsecchi, A.
Terrier, L. Bremond, A. Genries, S. Gauthier, and Y. Bergeron, 2012: Control of
the multimillennial wildfire size in boreal North America by spring climatic
conditions. Proceedings of the National Academy of Sciences of the United
States of America, 109(51), 20966-20970.
Alkama, R., M. Kageyama, and G. Ramstein, 2012: A sensitivity study to global
desertification in cold and warm climates: results from the IPSL OAGCM model.
Climate Dynamics, 38(7-8), 1629-1647.
Alkemade, R., M. van Oorschot, L. Miles, C. Nellemann, M. Bakkenes, and B. ten
Brink, 2009: GLOBIO3: a framework to investigate options for reducing global
terrestrial biodiversity loss. Ecosystems, 12(3), 374-390.
Allan, J.D., 2004: Landscapes and riverscapes: The influence of land use on stream
ecosystems. Annual Review of Ecology Evolution and Systematics, 35, 257-284.
Allen, C.D., A.K. Macalady, H. Chenchouni, D. Bachelet, N. McDowell, M. Vennetier,
T. Kitzberger, A. Rigling, D.D. Breshears, E.H. Hogg, P. Gonzalez, R. Fensham,
Z. Zhang, J. Castro, N. Demidova, J.H. Lim, G. Allard, S.W. Running, A. Semerci,
and N. Cobb, 2010: A global overview of drought and heat-induced tree
mortality reveals emerging climate change risks for forests. Forest Ecology and
Management, 259(4), 660-684.
Allen, J.R.M., T. Hickler, J.S. Singarayer, M.T. Sykes, P.J. Valdes, and B. Huntley, 2010:
Last glacial vegetation of northern Eurasia. Quaternary Science Reviews,
29(19-20), 2604-2618.
Alley, R.B., J. Marotzke, W.D. Nordhaus, J.T. Overpeck, D.M. Peteet, R.A. Pielke, R.T.
Pierrehumbert, P.B. Rhines, T.F. Stocker, L.D. Talley, and J.M. Wallace, 2003:
Abrupt climate change. Science, 299(5615), 2005-2010.
Anchukaitis, K.J. and M.N. Evans, 2010: Tropical cloud forest climate variability and
the demise of the Monteverde golden toad. Proceedings of the National
Academy of Sciences of the United States of America, 107(11), 5036-5040.
Anderegg, W.R.L., L.D.L. Anderegg, C. Sherman, and D.S. Karp, 2012: Effects of
widespread drought-induced aspen mortality on understory plants. Conservation
Biology, 26(6), 1082-1090.
Anderegg, W.R.L., J.M. Kane, and L.D.L. Anderegg, 2013a: Consequences of widespread
tree mortality triggered by drought and temperature stress. Nature Climate
Change, 3(1), 30-36.
Anderegg, W.R.L., L. Plavcová, L.D.L. Anderegg, U.G. Hacke, J.A. Berry, and C.B. Field,
2013b: Drought’s legacy: multiyear hydraulic deterioration underlies widespread
aspen forest die-off and portends increased future risk. Global Change Biology,
19(4), 1188-1196.
Anderson, J.T., A.M. Panetta, and T. Mitchell-Olds, 2012a: Evolutionary and ecological
responses to anthropogenic climate change. Plant Physiology, 160(4), 1728-
1740.
Anderson, J.T., D.W. Inouye, A.M. McKinney, R.I. Colautti, and T. Mitchell-Olds, 2012b:
Phenotypic plasticity and adaptive evolution contribute to advancing flowering
phenology in response to climate change. Proceedings of the Royal Society B,
279(1743), 3843-3852.
Anderson, R.G., J.G. Canadell, J.T. Randerson, R.B. Jackson, B.A. Hungate, D.D.
Baldocchi, G.A. Ban-Weiss, G.B. Bonan, K. Caldeira, L. Cao, N.S. Diffenbaugh,
K.R. Gurney, L.M. Kueppers, B.E. Law, S. Luyssaert, and T.L. O’Halloran, 2011:
Biophysical considerations in forestry for climate protection. Frontiers in Ecology
and the Environment, 9(3), 174-182.
Andreae, M.O., D. Rosenfeld, P. Artaxo, A.A. Costa, G.P. Frank, K.M. Longo, and M.A.F.
Silva-Dias, 2004: Smoking rain clouds over the Amazon. Science, 303, 1337-1342.
Andreu-Hayles, L., O. Planells, E. Gutiérrez, E. Muntan, G. Helle, K.J. Anchukaitis, and
G.H. Schleser, 2011: Long tree-ring chronologies reveal 20
th
century increases
in water-use efficiency but no enhancement of tree growth at five Iberian pine
forests. Global Change Biology, 17(6), 2095-2112.
Angassa, A. and G. Oba, 2008: Effects of management and time on mechanisms of
bush encroachment in southern Ethiopia. African Journal of Ecology, 46(2),
186-196.
Angeler, D.G. and W. Goedkoop, 2010: Biological responses to liming in boreal lakes:
an assessment using plankton, macroinvertebrate and fish communities. Journal
of Applied Ecology, 47(2), 478-486.
Angert, A.L., L.G. Crozier, L.J. Rissler, S.E. Gilman, J.J. Tewksbury, and A.J. Chunco,
2011: Do species’ traits predict recent shifts at expanding range edges? Ecology
Letters, 14(7), 677-689.
Angetter, L.S., S. Lotters, and D. Rodder, 2011: Climate niche shift in invasive species:
the case of the brown anole. Biological Journal of the Linnean Society, 104(4),
943-954.
Anyamba, A. and C.J. Tucker, 2005: Analysis of Sahelian vegetation dynamics using
NOAA-AVHRR NDVI data from 1981-2003. Journal of Arid Environments, 63(3),
596-614.
Aragão, L., Y. Malhi, N. Barbier, A. Lima, Y. Shimabukuro, L. Anderson, and S. Saatchi,
2008: Interactions between rainfall, deforestation and fires during recent years
in the Brazilian Amazonia. Philosophical Transactions of the Royal Society B,
363(1498), 1779-1785.
Araujo, M.B. and A.T. Peterson, 2012: Uses and misuses of bioclimatic envelope
modeling. Ecology, 93(7), 1527-1539.
Araujo, M.B., D. Alagador, M. Cabeza, D. Nogues-Bravo, and W. Thuiller, 2011: Climate
change threatens European conservation areas. Ecology Letters, 14(5), 484-492.
Archibald, S., D.P. Roy, B.W. van Wilgen, and R.J. Scholes, 2009: What limits fire? An
examination of drivers of burnt area in southern Africa. Global Change Biology,
15(3), 613-630.
Arenas, M., N. Ray, M. Currat, and L. Excoffier, 2012: Consequences of range
contractions and range shifts on molecular diversity. Molecular Biology and
Evolution, 29(1), 207-218.
Arismendi, I., S.L. Johnson, J.B. Dunham, R. Haggerty, and D. Hockman-Wert, 2012:
The paradox of cooling streams in a warming world: Regional climate trends
do not parallel variable local trends in stream temperature in the Pacific
continental United States. Geophysical Research Letters, 39, L10401,
doi:10.1029/2012GL051448.
Armenteras-Pascual, D., J. Retana-Alumbreros, R. Molowny-Horas, R.M. Roman-
Cuesta, F. Gonzalez-Alonso, and M. Morales-Rivas, 2011: Characterising
fire spatial pattern interactions with climate and vegetation in Colombia.
Agricultural and Forest Meteorology, 151(3), 279-289.
Arneth, A., S.P. Harrison, S. Zaehle, K. Tsigaridis, S. Menon, P.J. Bartlein, J. Feichter, A.
Korhola, M. Kulmala, D. O’Donnell, G. Schurgers, S. Sorvari, and T. Vesala, 2010:
Terrestrial biogeochemical feedbacks in the climate system. Nature Geoscience,
3(8), 525-532.
Arora, V.K. and A. Montenegro, 2011: Small temperature benefits provided by realistic
afforestation efforts. Nature Geoscience, 4(8), 514-518.
Arthington, A.H., S.E. Bunn, N.L. Poff, and R.J. Naiman, 2006: The challenge of
providing environmental flow rules to sustain river ecosystems. Ecological
Applications, 16(4), 1311-1318.
Arthington, A.H., R.J. Naiman, M.E. McClain, and C. Nilsson, 2010: Preserving the
biodiversity and ecological services of rivers: new challenges and research
opportunities. Freshwater Biology, 55(1), 1-16.
Ask, J., J. Karlsson, L. Persson, P. Ask, P. Bystrom, and M. Jansson, 2009: Terrestrial
organic matter and light penetration: Effects on bacterial and primary production
in lakes. Limnology and Oceanography, 54(6), 2034-2040.
Aubin, I., C.M. Garbe, S. Colombo, C.R. Drever, D.W. McKenney, C. Messier, J. Pedlar,
M.A. Saner, L. Venier, A.M. Wellstead, R. Winder, E. Witten, and C. Ste-Marie,
2011: Why we disagree about assisted migration: ethical implications of a key

330
Chapter 4 Terrestrial and Inland Water Systems
4
debate regarding the future of Canada’s forests. Forestry Chronicle, 87(6), 755-
765.
Aufdenkampe, A.K., E. Mayorga, P.A. Raymond, J.M. Melack, S.C. Doney, S.R. Alin,
R.E. Aalto, and K. Yoo, 2011: Riverine coupling of biogeochemical cycles between
land, oceans, and atmosphere. Frontiers in Ecology and the Environment, 9(1),
53-60.
Axford, Y., J.P. Briner, C.A. Cooke, D.R. Francis, N. Michelutti, G.H. Miller, J.P. Smol, E.K.
Thomas, C.R. Wilson, and A.P. Wolfe, 2009: Recent changes in a remote Arctic
lake are unique within the past 200,000 years. Proceedings of the National
Academy of Sciences of the United States of America, 106(44), 18443-18446.
Baccini, A., S.J. Goetz, W.S. Walker, N.T. Laporte, M. Sun, D. Sulla-Menashe, J. Hackler,
P.S.A. Beck, R. Dubayah, M.A. Fiedl, S. Samanta, and R.A. Houghton, 2012:
Estimated carbon dioxide emissions from tropical deforestation improved by
carbon-density maps. Nature Climate Change, 2(3), 182-185.
Bai, Z.G., D.L. Dent, L. Olsson, and M.E. Schaepman, 2008: Proxy global assessment
of land degradation. Soil Use and Management, 24(3), 223-234.
Bala, G., K. Caldeira, M. Wickett, T.J. Phillips, D.B. Lobell, C. Delire, and A. Mirin, 2007:
Combined climate and carbon-cycle effects of large-scale deforestation.
Proceedings of the National Academy of Sciences of the United States of
America, 104(16), 6550-6555.
Balch, J.K., D.C. Nepstad, P.M. Brando, L.M. Curran, O. Portela, O. de Carvalho, and P.
Lefebvre, 2008: Negative fire feedback in a transitional forest of southeastern
Amazonia. Global Change Biology, 14(10), 2276-2287.
Balian, E.V., H. Segers, C. Leveque, and K. Martens, 2008: The freshwater animal
diversity assessment: an overview of the results. Hydrobiologia, 595(1), 627-
637.
Balint, M., S. Domisch, C.H.M. Engelhardt, P. Haase, S. Lehrian, J. Sauer, K. Theissinger,
S.U. Pauls, and C. Nowak, 2011: Cryptic biodiversity loss linked to global climate
change. Nature Climate Change, 1(6), 313-318.
Ball, J.T., I.E. Woodrow, and J.A. Berry, 1987: A model predicting stomatal conductance
and its to the control of photosynthesis under different environmental conditions.
In: Progress in Photosynthesis Research: Proceedings of the VIIth International
Congress on Photosynthesis, Providence, Rhode Island, USA, August 10-15, Vol.
2 [ Biggins, I. (ed.)]. Martinus Nijhoff Publishers, Leiden, Netherlands, pp. 221-
224.
Barber, V.A., G.P. Juday, and B.P. Finney, 2000: Reduced growth of Alaskan white
spruce in the twentieth century from temperature-induced drought stress.
Nature, 405(6787), 668-673.
Barbet-Massin, M., W. Thuiller, and F. Jiguet, 2012: The fate of European breeding
birds under climate, land-use and dispersal scenarios. Global Change Biology,
18(3), 881-890.
Barbosa, I.C.R., I.H. Koehler, K. Auerswald, P. Lups, and S. Hans, 2010: Last-century
changes of alpine grassland water-use efficiency: a reconstruction through
carbon isotope analysis of a time-series of Capra ibex horns. Global Change
Biology, 16(4), 1171-1180.
Barbraud, C. and H. Weimerskirch, 2006: Antarctic birds breed later in response to
climate change. Proceedings of the National Academy of Sciences of the United
States of America, 103(16), 6248-6251.
Barbraud, C., M. Gavrilo, Y. Mizin, and H. Weimerskirch, 2011: Comparison of
emperor penguin declines between Pointe Geologie and Haswell Island over
the past 50 years. Antarctic Science, 23(5), 461-468.
Barclay, R.M.R., E.F. Baerwald, and J.C. Gruver, 2007: Variation in bat and bird
fatalities at wind energy facilities: assessing the effects of rotor size and tower
height. Canadian Journal of Zoology / Revue Canadienne De Zoologie, 85(3),
381-387.
Barlow, J. and C.A. Peres, 2008: Fire-mediated dieback and compositional cascade
in an Amazonian forest. Philosophical Transactions of the Royal Society B,
363(1498), 1787-1794.
Barnosky, A.D., N. Matzke, S. Tomiya, G.O.U. Wogan, B. Swartz, T.B. Quental, C.
Marshall, J.L. McGuire, E.L. Lindsey, K.C. Maguire, B. Mersey, and E.A. Ferrer,
2011: Has the Earth’s sixth mass extinction already arrived? Nature,
471(7336), 51-57.
Barnosky, A.D., E.A. Hadly, J. Bascompte, E.L. Berlow, J.H. Brown, M. Fortelius, W.M.
Getz, J. Harte, A. Hastings, P.A. Marquet, N.D. Martinez, A. Mooers, P. Roopnarine,
G. Vermeij, J.W. Williams, R. Gillespie, J. Kitzes, C. Marshall, N. Matzke, D.P.
Mindell, E. Revilla, and A.B. Smith, 2012: Approaching a state shift in Earth’s
biosphere. Nature, 486(7401), 52-58.
Bartholow, J.M., 2005: Recent water temperature trends in the lower Klamath River,
California. North American Journal of Fisheries Management, 25(1), 152-162.
Bartomeus, I., J.S. Ascher, D. Wagner, B.N. Danforth, S. Colla, S. Kornbluth, and R.
Winfree, 2011: Climate-associated phenological advances in bee pollinators
and bee-pollinated plants. Proceedings of the National Academy of Sciences
of the United States of America, 108(51), 20645-20649.
Bateman, B.L., J. VanDerWal, S.E. Williams, and C.N. Johnson, 2012: Biotic interactions
influence the projected distribution of a specialist mammal under climate
change. Diversity and Distributions, 18(9), 861-872.
Bateman, B.L., H.T. Murphy, A.E. Reside, K. Mokany, and J. VanDerWal, 2013:
Appropriateness of full-, partial- and no-dispersal scenarios in climate change
impact modelling. Diversity and Distributions, 19(10), 1224-1234.
Bathiany, S., M. Claussen, V. Brovkin, T. Raddatz, and V. Gayler, 2010: Combined
biogeophysical and biogeochemical effects of large-scale forest cover changes
in the MPI earth system model. Biogeosciences, 7(5), 1383-1399.
Battarbee, R.W., M. Kernan, and N. Rose, 2009: Threatened and stressed mountain
lakes of Europe: assessment and progress. Aquatic Ecosystem Health &
Management, 12(2), 118-128.
Beale, C.M. and J.J. Lennon, 2012: Incorporating uncertainty in predictive species
distribution modelling. Philosophical Transactions of the Royal Society B,
367(1586), 247-258.
Beaumont, L.J., A. Pitman, S. Perkins, N.E. Zimmermann, N.G. Yoccoz, and W. Thuiller,
2011: Impacts of climate change on the world’s most exceptional ecoregions.
Proceedings of the National Academy of Sciences of the United States of
America, 108(6), 2306-2311.
Beck, H.E., T.R. McVicar, A.I.L. van Dijk, J. Schellenkens, R.A.M. de Jeu, and L.A.
Bruijinzeel, 2011: Global evaluation of four AVHRR-NDVI data sets:
intercomparison and assessment against Landsat imagery. Remote Sensing of
Environment, 115(10), 2547-2563.
Beck, P.S.A. and S.J. Goetz, 2011: Satellite observations of high northern latitude
vegetation productivity changes between 1982 and 2008: ecological variability
and regional differences. Environmental Research Letters, 6(4), 045501,
doi:10.1088/1748-9326/6/4/045501.
Beck, P.S.A., G.P. Juday, A. Claire, W. Steve, S. Emily, H. Patricia, D.H. James, and S.J.
Goetz, 2011: Changes in forest productivity across Alaska are captured in
satellite and tree ring records. Ecology Letters, 14(4), 373-379.
Beerling, D.J. and C.P. Osborne, 2006: The origin of the savanna biome. Global
Change Biology, 12(11), 2023-2031.
Beier, C., I.K. Schmidt, and H.L. Kristensen, 2004: Effects of climate and ecosystem
disturbances on biogeochemical cycling in a semi-natural terrestrial ecosystem.
Water, Air and Soil Pollution: Focus, 4, 191-206.
Beier, C., B.A. Emmett, J. Penuelas, I.K. Schmidt, A. Tietema, M. Estiarte, P. Gundersen,
L. Llorens, T. Riis-Nielsen, A. Sowerby, and A. Gorissen, 2008: Carbon and nitro-
gen cycles in European ecosystems respond differently to global warming.
Science of the Total Environment, 407(1), 692-697.
Beilman, D.W., G.M. MacDonald, L.C. Smith, and P.J. Reimer, 2009: Carbon
accumulation in peatlands of West Siberia over the last 2000 years. Global
Biogeochemical Cycles, 23(1), GB1012, doi:10.1029/2007GB003112.
Bell, G., 2013: Evolutionary rescue and the limits of adaptation. Philosophical
Transactions of the Royal Society B, 368(1610), 20120080, doi:10.1098/
rstb.2012.0080.
Bell, G. and A. Gonzalez, 2009: Evolutionary rescue can prevent extinction following
environmental change. Ecology Letters, 12(9), 942-948.
Bellard, C., C. Bertelsmeier, P. Leadley, W. Thuiller, and F. Courchamp, 2012: Impacts
of climate change on the future of biodiversity. Ecology Letters, 15(4), 365-377.
Bellard, C., W. Thuiller, B. Leroy, P. Genovesi, M. Bakkenes, and F. Courchamp, 2013:
Will climate change promote future invasions? Global Change Biology, 19(12),
3740-3748.
Bellassen, V., N. Viovy, S. Luyssaert, G. Le Maire, M.J. Schelhaas, and P. Ciais, 2011:
Reconstruction and attribution of the carbon sink of European forests between
1950 and 2000. Global Change Biology, 17(11), 3274-3292.
Belyazid, S., D. Kurz, S. Braun, H. Sverdrup, B. Rihm, and J.P. Hettelingh, 2011: A
dynamic modelling approach for estimating critical loads of nitrogen based on
pliant community changes under a changing climate. Environmental Pollution,
159(3), 789-801.
Bentz, B.J., J. Régnière, C.J. Fettig, E.M. Hansen, J.L. Hayes, J.A. Hicke, R.G. Kelsey, J.F.
Negrón, and S.J. Seybold, 2010: Climate change and bark beetles of the Western
United States and Canada: direct and indirect effects. Bioscience, 60(8), 602-613.
Berg, M.P., E.T. Kiers, G. Driessen, M. Van Der Heijden, B.W. Kooi, F. Kuenen, M. Liefting,
H.A. Verhoef, and J. Ellers, 2010: Adapt or disperse: understanding species
persistence in a changing world. Global Change Biology, 16(2), 587-598.

331
Terrestrial and Inland Water Systems Chapter 4
4
Bergeron, Y., D. Cyr, M.P. Girardin, and C. Carcaillet, 2010: Will climate change drive
21
st
century burn rates in Canadian boreal forest outside of its natural variability:
collating global climate model experiments with sedimentary charcoal data.
International Journal of Wildland Fire, 19(8), 1127-1139.
Bergström, A.K. and M. Jansson, 2006: Atmospheric nitrogen deposition has caused
nitrogen enrichment and eutrophication of lakes in the northern hemisphere.
Global Change Biology, 12, 635-643.
Berkes, F., J. Colding, and C. Folke (eds.), 2003: Navigating Social-Ecological Systems.
Building Resilience for Complexity and Change. Cambridge University Press,
Cambridge, UK, 393 pp.
Bernacchi, C.J., A.D.B. Leakey, L.E. Heady, P.B. Morgan, F.G. Dohleman, J.M. McGrath,
K.M. Gillespie, V.E. Wittig, A. Rogers, S.P. Long, and D.R. Ort, 2006: Hourly and
seasonal variation in photosynthesis and stomatal conductance of soybean
grown at future CO
2
and ozone concentrations for 3 years under fully open-air
field conditions. Plant Cell and Environment, 29(11), 2077-2090.
Bernhardt, E.L., T.N. Hollingsworth, and F.S. Chapin III, 2011: Fire severity mediates
climate-driven shifts in understorey community composition of black spruce
stands of interior Alaska. Journal of Vegetation Science, 22(1), 32-44.
Bertaux, D., D. Reale, A.G. McAdam, and S. Boutin, 2004: Keeping pace with fast
climate change: can Arctic life count on evolution? Integrative and Comparitive
Biology, 44(2), 140-151.
Bertelsmeier, C., G. Luque, and F. Courchamp, 2012: Global warming may freeze the
invasion of big-headed ants. Biological Invasions, 15(7), 1561-1572.
Bertrand, R., J. Lenoir, C. Piedallu, G. Riofrio-Dillon, P. de Ruffray, C. Vidal, J.C. Pierrat,
and J.C. Gegout, 2011: Changes in plant community composition lag behind
climate warming in lowland forests. Nature, 479(7374), 517-520.
Bertzky, M., B. Dickson, R. Galt, E. Glen, M. Harley, N. Hodgson, G. Keder, I. Lysenko,
M. Pooley, C. Ravilious, T. Sajwaj, R. Schiopu, Y. de Soye, and G. Tucker, 2010:
Impacts of Climate Change and Selected Renewable Energy Infrastructures on
EU Biodiversity and the Natura 2000 Network. Summary Report. European
Commission and International Union for Conservation of Nature, Brussels.
http://ec.europa.eu/environment/nature/climatechange/pdf/study.pdf.
Betts, R.A., N.W. Arnell, P. Boorman, S.E. Cornell, J.I. House, N.R. Kaye, M.P. McCarthy,
D. McNeall, M.G. Sanderson, and A.J. Wiltshire, 2012: Climate change impacts
and adaptation: an earth system view. In: Understanding the Earth System:
Global Change Science for Application [Cornell, S., C. Prentice, J. House, and C.
Downy (eds.)]. Cambridge University Press, Cambridge, UK, pp. 160-201.
Betts, R.A., N. Golding, P. Gonzalez, J. Gornall, R. Kahana, G. Kay, L. Mitchell, and A.
Wiltshire, 2013: Climate and land use change impacts on global terrestrial
ecosystems, fire, and river flows in the HadGEM2-ES Earth System Model using
the Representative Concentration Pathways. Biogeosciences Discussions, 10,
6171-6223. doi:10.5194/bgd-10-6171-2013.
Bhatt, U.S., D.A. Walker, M.K. Raynolds, J.C. Comiso, H.E. Epstein, G.S. Jia, R. Gens,
J.E. Pinzon, C.J. Tucker, C.E. Tweedie, and P.J. Webber, 2010: Circumpolar Arctic
tundra vegetation change is linked to sea ice decline. Earth Interactions, 14, 8,
doi:10.1175/2010EI315.1.
Biesmeijer, J.C., S.P.M. Roberts, M. Reemer, R. Ohlemuller, M. Edwards, T. Peeters,
A.P. Schaffers, S.G. Potts, R. Kleukers, C.D. Thomas, J. Settele, and W.E. Kunin,
2006: Parallel declines in pollinators and insect-pollinated plants in Britain and
The Netherlands. Science, 313(5785), 351-354.
Biggs, D., R. Biggs, V. Dakos, R.J. Scholes, and M. Schoon, 2011: Are we entering an
era of concatenated global crises? Ecology and Society, 16(2), 27, www.ecology
andsociety.org/vol16/iss2/art27/.
Biggs, R., S.R. Carpenter, and W.A. Brock, 2009: Turning back from the brink: detecting
an impending regime shift in time to avert it. Proceedings of the National
Academy of Sciences of the United States of America, 106(3), 826-831.
Birdsey, R.A., K.S. Pregitzer, and A. Lucier, 2006: Forest carbon management in the
United States: 1600-2100. Journal of Environmental Quality, 35(4), 1461-
1469.
Bleeker, A., W.K. Hicks, E. Dentener, J. Galloway, and J.W. Erisman, 2011: N deposition
as a threat to the World’s protected areas under the Convention on Biological
Diversity. Environmental Pollution, 159(10), 2280-2288.
Blok, D., M.M.P.D. Heijmans, G. Schaepman-Strub, A.V. Kononov, T.C. Maximov, and
F. Berendse, 2010: Shrub expansion may reduce summer permafrost thaw in
Siberian tundra. Global Change Biology, 16(4), 1296-1305.
Bloor, J., P. Pichon, R. Falcimagne, P. Leadley, and J.-F. Soussana, 2010: Effects of
warming, summer drought, and CO
2
enrichment on aboveground biomass
production, flowering phenology, and community structure in an upland
grassland ecosystem. Ecosystems, 13(6), 888-900.
BMT WBM, 2010: Kakadu – Vulnerability to Climate Change Impacts. A report to the
Australian Government Department of Climate Change and Energy Efficiency.
Australian Government, Department of Climate Change and Energy Efficiency,
Canberra, ACT, Australia, 226 pp.
Bobbink, R., K. Hicks, J. Galloway, T. Spranger, R. Alkemade, M. Ashmore, M. Bustamante,
S. Cinderby, E. Davidson, F. Dentener, B. Emmett, J.W. Erisman, M. Fenn, F.
Gilliam, A. Nordin, L. Pardo, and W. De Vries, 2010: Global assessment of nitrogen
deposition effects on terrestrial plant diversity: a synthesis. Ecological Applications,
20(1), 30-59.
Bockheim, J., G. Vieira, M. Ramos, J. Lopez-Martinez, E. Serrano, M. Guglielmin, K.
Wilhelm, and A. Nieuwendam, 2013: Climate warming and permafrost dynamics
in the Antarctic Peninsula region. Global and Planetary Change, 100, 215-223.
Boggs, C.L. and D.W. Inouye, 2012: A single climate driver has direct and indirect
effects on insect population dynamics. Ecology Letters, 15(5), 502-508.
Boisvenue, C. and S.W. Running, 2006: Impacts of climate change on natural forest
productivity - evidence since the middle of the 20
th
century. Global Change
Biology, 12(5), 862-882.
Bolte, A. and B. Degen, 2010: Forest adaptation to climate change – options and
limitations. Landbauforschung, 60(3), 111-117.
Bolte, A., C. Ammer, M. Lof, P. Madsen, G.J. Nabuurs, P. Schall, P. Spathelf, and J. Rock,
2009: Adaptive forest management in central Europe: Climate change impacts,
strategies and integrative concept. Scandinavian Journal of Forest Research,
24(6), 473-482.
Bolte, A., L. Hilbrig, B. Grundmann, F. Kampf, J. Brunet, and A. Roloff, 2010: Climate
change impacts on stand structure and competitive interactions in a southern
Swedish spruce-beech forest. European Journal of Forest Research, 129(3),
261-276.
Bomhard, B., D.M. Richardson, J.S. Donaldson, G.O. Hughes, G.F. Midgley, D.C.
Raimondo, A.G. Rebelo, M. Rouget, and W. Thuiller, 2005: Potential impacts of
future land use and climate change on the Red List status of the Proteaceae in
the Cape Floristic Region, South Africa. Global Change Biology, 11(9), 1452-1468.
Bonan, G.B., 2008: Forests and climate change: forcings, feedbacks, and the climate
benefits of forests. Science, 320(5882), 1444-1449.
Bond, N.R., P.S. Lake, and A.H. Arthington, 2008: The impacts of drought on freshwater
ecosystems: an Australian perspective. Hydrobiologia, 600(1), 3-16.
Bond, W.J. and J.J. Midgley, 2001: Ecology of sprouting in woody plants: the persistence
niche. Trends in Ecology & Evolution, 16(1), 45-51.
Bond, W.J. and G.F. Midgley, 2012: Carbon dioxide and the uneasy interactions of
trees and savannah grasses. Philosophical Transactions of the Royal Society B,
367(1588), 601-612.
Bond-Lamberty, B. and A. Thomson, 2010: Temperature-associated increases in the
global soil respiration record. Nature, 464(7288), 579-582.
Bond-Lamberty, B., S.D. Peckham, D.E. Ahl, and S.T. Gower, 2007: Fire as the dominant
driver of central Canadian boreal forest carbon balance. Nature, 450(7166),
89-92.
Bonfils, C.J.W., T.J. Phillips, D.M. Lawrence, P. Cameron-Smith, W.J. Riley, and Z.M.
Subin, 2012: On the influence of shrub height and expansion on northern high
latitude climate. Environmental Research Letters, 7(1), 015503, doi:10.1088/
1748-9326/7/1/015503.
Bontemps, J.D., J.C. Herve, J.M. Leban, and J.F. Dhote, 2011: Nitrogen footprint in a
long-term observation of forest growth over the twentieth century. Trees –
Structure and Function, 25(2), 237-251.
Booker, K., L. Huntsinger, J.W. Bartolome, N.F. Sayre, and W. Stewart, 2013: What can
ecological science tell us about opportunities for carbon sequestration on arid
rangelands in the United States? Global Environmental Change: Human and
Policy Dimensions, 23(1), 240-251.
Booth, R.K., S.T. Jackson, S.L. Forman, J.E. Kutzbach, E.A. Bettis, J. Kreig, and D.K.
Wright, 2005: A severe centennial-scale drought in mid-continental North
America 4200 years ago and apparent global linkages. Holocene, 15(3), 321-328.
Bosio, J., M. Johansson, T.V. Callaghan, B. Johansen, and T.R. Christensen, 2012: Future
vegetation changes in thawing subarctic mires and implications for greenhouse
gas exchange – a regional assessment. Climatic Change, 115(2), 379-398.
Both, C., S. Bouwhuis, C.M. Lesselis, and M.E. Visser, 2006: Climate change and
population declines in a long-distance migratory bird. Nature, 441(7089), 81-
83.
Both, C., C.A.M. Van Turnhout, R.G. Bijlsma, H. Siepel, A.J. Van Strien, and R.P.B. Foppen,
2010: Avian population consequences of climate change are most severe for
long-distance migrants in seasonal habitats. Proceedings of the Royal Society
B, 277(1685), 1259-1266.

332
Chapter 4 Terrestrial and Inland Water Systems
4
Botkin, D.B., H. Saxe, M.B. Araujo, R. Betts, R.H.W. Bradshaw, T. Cedhagen, P. Chesson,
T.P. Dawson, J.R. Etterson, D.P. Faith, S. Ferrier, A. Guisan, A.S. Hansen, D.W. Hilbert,
C. Loehle, C. Margules, M. New, M.J. Sobel, and D.R.B. Stockwell, 2007: Forecasting
the effects of global warming on biodiversity. BioScience, 57(3), 227-236.
Boutton, T.W., J.D. Liao, T.R. Filley, and S.R. Archer, 2009: Belowground carbon storage
and dynamics accompanying woody plant encroachment in a subtropical
savanna. Soil Carbon Sequestration and the Greenhouse Effect [Lal, R. and R.
Follett (eds.)]. 2
nd
edn., Soil Science Society of America, Inc., Madison, WI, USA,
pp. 181-205.
Bowman, D., J.K. Balch, P. Artaxo, W.J. Bond, J.M. Carlson, M.A. Cochrane, C.M.
D’Antonio, R.S. DeFries, J.C. Doyle, S.P. Harrison, F.H. Johnston, J.E. Keeley, M.A.
Krawchuk, C.A. Kull, J.B. Marston, M.A. Moritz, I.C. Prentice, C.I. Roos, A.C. Scott,
T.W. Swetnam, G.R. van der Werf, and S.J. Pyne, 2009: Fire in the Earth system.
Science, 324(5926), 481-484.
Bowman, D.M.J.S., B.P. Murphy, and D.S. Banfai, 2011: Has global environmental
change caused monsoon rainforests to expand in the Australian monsoon
tropics? Landscape Ecology, 25(8), 1247-1260.
Bradley, B.A., M. Oppenheimer, and D.S. Wilcove, 2009: Climate change and plant
invasions: restoration opportunities ahead? Global Change Biology, 15(6),
1511-1521.
Bradley, B.A., D.M. Blumenthal, D.S. Wilcove, and L.H. Ziska, 2010: Predicting plant
invasions in an era of global change. Trends in Ecology & Evolution, 25(5), 310-
318.
Brandão, R.A. and A.F.B. Araújo, 2007: Changes in anuran species richness and
abundance resulting from hydroelectric dam flooding in central Brazil.
Biotropica, 40(2), 263-266.
Brando, P.M., S.J. Goetz, A. Baccini, D.C. Nepstad, P.S.A. Beck, and M.C. Christman,
2010: Seasonal and interannual variability of climate and vegetation indices
across the Amazon. Proceedings of the National Academy of Sciences of the
United States of America, 107(33), 14685-14690.
Brando, P.M., D.C. Nepstad, J.K. Balch, B. Bolker, M.C. Christman, M.T. Coe, and F.E.
Putz, 2012: Fire-induced tree mortality in a neotropical forest: the roles of bark
traits, tree size, wood density and fire behavior. Global Change Biology, 18(2),
630-641.
Brasier, C. and J. Webber, 2010: Plant pathology: Sudden larch death. Nature,
466(7308), 824-825.
Breshears, D.D., 2006: The grassland-forest continuum: trends in ecosystem properties
for woody plant mosaics? Frontiers in Ecology and the Environment, 4(2), 96-104.
Breshears, D.D., N.S. Cobb, P.M. Rich, K.P. Price, C.D. Allen, R.G. Balice, W.H. Romme,
J.H. Kastens, M.L. Floyd, J. Belnap, J.J. Anderson, O.B. Myers, and C.W. Meyer,
2005: Regional vegetation die-off in response to global-change-type drought.
Proceedings of the National Academy of Sciences of the United States of
America, 102(42), 15144-15148.
Bridle, J.R., J. Polechova, M. Kawata, and R.K. Butlin, 2010: Why is adaptation prevented
at ecological margins? New insights from individual-based simulations. Ecology
Letters, 13(4), 485-494.
Briffa, K.R., V.V. Shishov, T.M. Melvin, E.A. Vaganov, H. Grudd, R.M. Hantemirov, M.
Eronen, and M.M. Naurzbaev, 2008: Trends in recent temperature and radial
tree growth spanning 2000 years across northwest Eurasia. Philosophical
Transactions of the Royal Society B, 363(1501), 2271-2284.
Brink, V.C., 1959: A directional change in the subalpine forest-heath ecotone in
Garibaldi Park, British Columbia. Ecology, 40(1), 10-16.
Brisson, J., S. de Blois, and C. Lavoie, 2010: Roadside as invasion pathway for common
reed (Phragmites australis). Invasive Plant Science and Management, 3(4), 506-
514.
Brittain, C., R. Bommarco, M. Vighi, S. Barmaz, J. Settele, and S.G. Potts, 2010a:
The impact of an insecticide on insect flower visitation and pollination in an
agricultural landscape. Agricultural and Forest Entomology, 12(3), 259-266.
Brittain, C.A., M. Vighi, R. Bommarco, J. Settele, and S.G. Potts, 2010b: Impacts of a
pesticide on pollinator species richness at different spatial scales. Basic and
Applied Ecology, 11(2), 106-115.
Britton, A.J., C.M. Beale, W. Towers, and R.L. Hewison, 2009: Biodiversity gains and
losses: Evidence for homogenisation of Scottish alpine vegetation. Biological
Conservation, 142(8), 1728-1739.
Britton, J.R., J. Cucherousset, G.D. Davies, M.J. Godard, and G.H. Copp, 2010: Non-
native fishes and climate change: predicting species responses to warming
emperatures in a temperate region. Freshwater Biology, 55(5), 1130-1141.
Broadmeadow, M.S.J., D. Ray, and C.J.A. Samuel, 2005: Climate change and the
future for broadleaved tree species in Britain. Forestry, 78(2), 145-161.
Broennimann, O., U.A. Treier, H. Muller-Scharer, W. Thuiller, A.T. Peterson, and A.
Guisan, 2007: Evidence of climatic niche shift during biological invasion.
Ecology Letters, 10(8), 701-709.
Brommer, J.E., A. Lehikoinen, and J. Valkama, 2012: The breeding ranges of central
European and Arctic bird species move poleward. PLoS One, 7(9), e43648,
doi:10.1371/journal.pone.0043648.
Bronson, D.R., S.T. Gower, M. Tanner, and I. Van Herk, 2009: Effect of ecosystem
warming on boreal black spruce bud burst and shoot growth. Global Change
Biology, 15(6), 1534-1543.
Brook, B.W., 2008: Synergies between climate change, extinctions and invasive
vertebrates. Wildlife Research, 35(3), 249-252.
Brook, B.W. and D.M.J.S. Bowman, 2006: Postcards from the past: charting the
landscape-scale conversion of tropical Australian savanna to closed forest
during the 20
t
h
century. Landscape Ecology, 21(8), 1253-1266.
Brook, B.W., N.S. Sodhi, and C.J.A. Bradshaw, 2008: Synergies among extinction
drivers under global change. Trends in Ecology & Evolution, 23(8), 453-460.
Brook, B.W., E.C. Ellis, M.P. Perring, A.W. Mackay, and L. Blomqvist, 2013: Does the
terrestrial biosphere have planetary tipping points? Trends in Ecology &
Evolution, 28(7), 396-401.
Brouwers, N., J. Mercer, T. Lyons, P. Poot, E. Veneklaas, and G. Hardy, 2012: Climate
and landscape drivers of tree decline in a Mediterranean ecoregion. Ecology
and Evolution, 3(1), 67-79.
Brouwers, N., G. Matusick, K. Ruthrof, T. Lyons, and G. Hardy, 2013: Landscape-scale
assessment of tree crown dieback following extreme drought and heat in a
Mediterranean eucalypt forest ecosystem. Landscape Ecology, 28(1), 69-80.
Brovkin, V., L. Boysen, T. Raddatz, V. Gayler, A. Loew, and M. Claussen, 2013:
Evaluation of vegetation cover and land-surface albedo in MPI-ESM CMIP5
simulations. Journal of Advances in Modeling Earth Systems, 5(1), 48-57.
Brown, C.D., 2010: Tree-line dynamics: adding fire to climate change prediction.
Arctic, 63(4), 488-492.
Brown, L.E., D.M. Hannah, and A.M. Milner, 2007: Vulnerability of alpine stream
biodiversity to shrinking glaciers and snowpacks. Global Change Biology, 13(5),
958-966.
Brusca, R.C., J.F. Wiens, W.M. Meyer, J. Eble, K. Franklin, J.T. Overpeck, and M. W., 2013:
Dramatic response to climate change in the Southwest: Robert Whittaker’s
1963 Arizona Mountain plant transect revisited. Ecology and Evolution, 3(10),
3307-3319.
Bryant, M.D., 2009: Global climate change and potential effects on Pacific salmonids
in freshwater ecosystems of southeast Alaska. Climatic Change, 95(1-2), 169-
193.
Buckley, J., R.K. Butlin, and J.R. Bridle, 2012: Evidence for evolutionary change
associated with the recent range expansion of the British butterfly, Aricia
agestis, in response to climate change. Molecular Ecology, 21(2), 267-280.
Buckley, L.B., M.C. Urban, M.J. Angilletta, L.G. Crozier, L.J. Rissler, and M.W. Sears,
2010: Can mechanism inform species’ distribution models? Ecology Letters,
13(8), 1041-1054.
Buongiorno, J., R. Raunikar, and S. S. A. Zhu, 2011: Consequences of increasing
bioenergy demand on wood and forests: An application of the Global Forest
Products Model. Journal of Forest Economics, 17(2), 214-229.
Buisson, L. and G. Grenouillet, 2009: Contrasted impacts of climate change on stream
fish assemblages along an environmental gradient. Diversity and Distributions,
15(4), 613-626.
Buisson, L., W. Thuiller, S. Lek, P. Lim, and G. Grenouillet, 2008: Climate change
hastens the turnover of stream fish assemblages. Global Change Biology,
14(10), 2232-2248.
Buitenwerf, R., W.J. Bond, N. Stevens, and W.W. Trollope, 2012: Increased tree
densities in two South African savannas: > 50 years of data suggests CO
2
as a
driver. Global Change Biology, 18(2), 675-684.
Bunn, S.E. and A.H. Arthington, 2002: Basic principles and ecological consequences
of altered flow regimes for aquatic biodiversity. Environmental Management,
30(4), 492-507.
Burgmer, T., H. Hillebrand, and M. Pfenninger, 2007: Effects of climate-driven
temperature changes on the diversity of freshwater macroinvertebrates.
Oecologia, 151(1), 93-103.
Burkhead, N.M., 2012: Extinction rates in North American freshwater fishes, 1900-
2010. BioScience, 62(9), 798-808.
Burkle, L.A., J.C. Marlin, and T.M. Knight, 2013: Plant-pollinator interactions over
120 Years: loss of species, co-occurrence, and function. Science, 339(6127),
1611-1615.

333
Terrestrial and Inland Water Systems Chapter 4
4
Burrows, M.T., D.S. Schoeman, L.B. Buckley, P. Moore, E.S. Poloczanska, K.M. Brander,
C. Brown, J.F. Bruno, C.M. Duarte, B.S. Halpern, J. Holding, C.V. Kappel, W.
Kiessling, M.I. O’Connor, J.M. Pandolfi, C. Parmesan, F.B. Schwing, W.J. Sydeman,
and A.J. Richardson, 2011: The pace of shifting climate in marine and terrestrial
ecosystems. Science, 334(6056), 652-655.
Burton, O.J., B.L. Phillips, and J.M.J. Travis, 2010: Trade-offs and the evolution of life-
histories during range expansion. Ecology Letters, 13(10), 1210-1220.
Bustamante, H.M., L.J. Livo, and C. Carey, 2010: Effects of temperature and hydric
environment on survival of the Panamanian Golden Frog infected with a
pathogenic chytrid fungus. Integrative Zoology, 5(2), 143-153.
Buswell, J.M., A.T. Moles, and S. Hartley, 2011: Is rapid evolution common in
introduced plant species? Journal of Ecology, 99(1), 214-224.
Bütof, A., L.R. von Riedmatten, C.F. Dormann, M. Scherer-Lorenzen, E. Welk, and H.
Bruelheide, 2012: The responses of grassland plants to experimentally simulated
climate change depend on land use and region. Global Change Biology, 18(1),
127-137.
Butt, N., P.A. de Oliveira, and M.H. Costa, 2011: Evidence that deforestation affects
the onset of the rainy season in Rondonia, Brazil. Journal of Geophysical
Research: Atmospheres, 116(D11), D11120, doi:10.1029/2010JD015174.
Cabral, A.C., J.M. Miguel, A.J. Rescia, M.F. Schmitz, and F.D. Pineda, 2009: Shrub
encroachment in Argentinean savannas. Journal of Vegetation Science, 14(2),
145-152.
Cadotte, M.W., 2006: Dispersal and species diversity: a meta-analysis. American
Naturalist, 167(6), 913-924.
Caesar, J., E. Palin, S. Liddicoat, J. Lowe, E. Burke, A. Pardaens, M. Sanderson, and R.
Kahana, 2013: Response of the HadGEM2 Earth System Model to future
greenhouse gas emissions pathways to the year 2300. Journal of Climate,
26(10), 3275-3284.
Cahill, A.E., M.E. Aiello-Lammens, M.C. Fisher-Reid, X. Hua, C.J. Karanewsky, H.Y. Ryu,
G.C. Sbeglia, F. Spagnolo, J.B. Waldron, O. Warsi, and J.J. Wiens, 2013: How does
climate change cause extinction? Proceedings of the Royal Society B,
280(1750), 20121890, doi:10.1098/rspb.2012.1890.
Cailleret, M., M. Nourtier, A. Amm, M. Durand-Gillmann, and H. Davi, 2013: Drought-
induced decline and mortality of silver fir differ among three sites in Southern
France. Annals of Forest Science, doi:10.1007/s13595-013-0265-0.
Caissie, D., 2006: The thermal regime of rivers: a review. Freshwater Biology, 51(8),
1389-1406.
Caldow, R.W.G., R.A. Stillman, S.E.A. le V. dit Durell, A.D. West, S. McGrorty, J.D. Goss-
Custard, P.J. Wood, and J. Humphreys, 2007: Benefits to shorebirds from invasion
of a non-native shellfish. Proceedings of the Royal Society B, 274(1616), 1449-
1455.
Cameron, A., 2012: Refining risk estimates using models. In: Saving a Million Species:
Extinction Risk from Climate Change [Hannah, L. (ed.)]. Island Press, Washington,
DC, USA, pp. 41-72.
Canadell, J.G. and M.R. Raupach, 2008: Managing forests for climate change
mitigation. Science, 320(5882), 1456-1457.
Canadell, J.G., C. Le Quéré, M.R. Raupach, C.B. Field, E.T. Buitenhuis, P. Ciais, T.J.
Conway, N.P. Gillett, R.A. Houghton, and G. Marland, 2007: Contributions to
accelerating atmospheric CO
2
growth from economic activity, carbon intensity,
and efficiency of natural sinks. Proceedings of the National Academy of
Sciences of the United States of America, 104(47), 18866-18870.
Canfield, D.E., A.N. Glazer, and P.G. Falkowski, 2010: The evolution and future of
Earth’s nitrogen cycle. Science, 330(6001), 192-196.
Cannone, N., S. Sgorbati, and M. Guglielmin, 2007: Unexpected impacts of climate
change on alpine vegetation. Frontiers in Ecology and the Environment, 5(7),
360-364.
Cannone, N., G. Diolaiuti, M. Guglielmin, and C. Smiraglia, 2008: Accelerating climate
change impacts on alpine glacier forefield ecosystems in the European Alps.
Ecological Applications, 18(3), 637-648.
Capon, S.J., 2007: Effects of flooding on seedling emergence from the soil seed bank
of a large desert floodplain. Wetlands, 27(4), 904-914.
Capon, S.J., L.E. Chambers, R. Mac Nally, R.J. Naiman, P. Davies, N. Marshall, J. Pittock,
M. Reid, T. Capon, M. Douglas, J. Catford, D.S. Baldwin, M. Stewardson, J.
Roberts, M. Parsons, and S.E. Williams, 2013: Riparian ecosystems in the 21
st
century: hotspots for climate change adaptation? Ecosystems, 16(3), 359-381.
Carmo, J.B.d., E.R. de Sousa Neto, P.J. Duarte-Neto, J.P.H.B. Ometto, and L.A. Martinelli,
2012: Conversion of the coastal Atlantic forest to pasture: Consequences for
the nitrogen cycle and soil greenhouse gas emissions. Agriculture, Ecosystems
& Environment, 148(0), 37-43.
Carnicer, J., M. Coll, M. Ninyerola, X. Pons, G. Sanchez, and J. Penuelas, 2011:
Widespread crown condition decline, food web disruption, and amplified tree
mortality with increased climate change-type drought. Proceedings of the
National Academy of Sciences of the United States of America, 108(4), 1474-
1478.
Cavagnaro, T.R., R.M. Gleadow, and R.E. Miller, 2011: Plant nutrient acquisition and
utilisation in a high carbon dioxide world. Functional Plant Biology, 38(2), 87-
96.
CBD, 2012: Geoengineering in Relation to the Convention on Biological Diversity:
Technical and Regulatory Matters. CBD Technical Series Series 66, Secretariat
of the Convention on Biological Diversity, Montreal, Cannada, 152 pp.
Chapin III, F.S., M. Sturm, M.C. Serreze, J.P. McFadden, J.R. Key, A.H. Lloyd, A.D.
McGuire, T.S. Rupp, A.H. Lynch, J.P. Schimel, J. Beringer, W.L. Chapman, H.E.
Epstein, E.S. Euskirchen, L.D. Hinzman, G. Jia, C.L. Ping, K.D. Tape, C.D.C. Thompson,
D.A. Walker, and J.M. Welker, 2005: Role of land-surface changes in Arctic
summer warming. Science, 310(5748), 657-660.
Charru, M., I. Seynave, F. Morneau, and J.D. Bontemps, 2010: Recent changes in forest
productivity: an analysis of national forest inventory data for common beech
(Fagus sylvatica L.) in north-eastern France. Forest Ecology and Management,
260(5), 864-874.
Chaturvedi, R.K., R. Gopalakrishnan, M. Jayaraman, G. Bala, N.V. Joshi, R. Sukumar,
and N.H. Ravindranath, 2011: Impact of climate change on Indian forests: a
dynamic vegetation modeling approach. Mitigation and Adaptation Strategies
for Global Change, 16(2), 119-142.
Cheaib, A., V. Badeau, J. Boe, I. Chuine, C. Delire, E. Dufrêne, C. François, E.S. Gritti,
M. Legay, C. Pagé, W. Thuiller, N. Viovy, and P. Leadley, 2012: Climate change
impacts on tree ranges: model intercomparison facilitates understanding and
quantification of uncertainty. Ecology Letters, 15(6), 533-544.
Chen, I.-C., H.J. Shiu, S. Benedick, J.D. Holloway, V.K. Cheye, H.S. Barlow, J.K. Hill, and
C.D. Thomas, 2009: Elevation increases in moth assemblages over 42 years on
a tropical mountain. Proceedings of the National Academy of Sciences of the
United States of America, 106(5), 1479-1483.
Chen, I.-C., J.K. Hill, R. Ohlemüller, D.B. Roy, and C.D. Thomas, 2011: Rapid range shifts
of species associated with high levels of climate warming. Science, 333(6045),
1024-1026.
Chessman, B.C., 2009: Climatic changes and 13-year trends in stream macroinvertebrate
assemblages in New South Wales, Australia. Global Change Biology, 15(11),
2791-2802.
Chevin, L.M., R. Lande, and G.M. Mace, 2010: Adaptation, plasticity, and extinction
in a changing environment: towards a predictive theory. PLoS Biology, 8(4),
e1000357, doi:10.1371/journal.pbio.1000357.
Chiba, S. and K. Roy, 2011: Selectivity of terrestrial gastropod extinctions on an
oceanic archipelago and insights into the anthropogenic extinction process.
Proceedings of the National Academy of Sciences of the United States of
America, 108(23), 9496-9501.
Chisholm, R.A., 2010: Trade-offs between ecosystem services: Water and carbon in
a biodiversity hotspot. Ecological Economics, 69(10), 1973-1987.
Chmura, D.J., P.D. Anderson, G.T. Howe, C.A. Harrington, J.E. Halofsky, D.L. Peterson,
D.C. Shaw, and J.B. St. Clair, 2011: Forest responses to climate change in the
northwestern United States: ecophysiological foundations for adaptive
management. Forest Ecology and Management, 261(7), 1121-1142.
Choat, B., S. Jansen, T.J. Brodribb, H. Cochard, S. Delzon, R. Bhaskar, S.J. Bucci,
T.S. Feild, S.M. Gleason, U.G. Hacke, A.L. Jacobsen, F. Lens, H. Maherali, J.
Martinez-Vilalta, S. Mayr, M. Mencuccini, P.J. Mitchell, A. Nardini, J. Pittermann,
R.B. Pratt, J.S. Sperry, M. Westoby, I.J. Wright, and A.E. Zanne, 2012: Global
convergence in the vulnerability of forests to drought. Nature, 491(7426), 752-
755.
Chown, S.L., A.A. Hoffmann, T.N. Kristensen, M.J. Angilletta, N.C. Stenseth, and C.
Pertoldi, 2010: Adapting to climate change: a perspective from evolutionary
physiology. Climate Research, 43(1-2), 3-15.
Chown, S.L., A.H.L. Huiskes, N.J.M. Gremmen, J.E. Lee, A. Terauds, K. Crosbie, Y. Frenot,
K.A. Hughes, S. Imura, K. Kiefer, M. Lebouvier, B. Raymond, M. Tsujimoto, C. Ware,
B. van den Vijver, and D.M. Bergstrom, 2012: Continent-wide risk assessment
for the establishment of nonindigenous species in Antarctica. Proceedings of
the National Academy of Sciences of the United States of America, 109(13),
4938-4943.
Christie, P. and M. Sommerkorn, 2012: RaCeR: Rapid Assessment of Circum-Arctic
Ecosystem Resilience. WWF Global Arctic Programme, World Wildlife Fund
(WWF), Ottawa, Canada, 70 pp.

334
Chapter 4 Terrestrial and Inland Water Systems
4
Chuine, I., X. Morin, L. Sonie, C. Collin, J. Fabreguettes, D. Degueldre, J.L. Salager, and
J. Roy, 2012: Climate change might increase the invasion potential of the alien
C4 grass Setaria parviflora (Poaceae) in the Mediterranean Basin. Diversity and
Distributions, 18(7), 661-672.
Churkina, G., S. Zaehle, J. Hughes, N. Viovy, Y. Chen, M. Jung, B.W. Heumann, N.
Ramankutty, M. Heimann, and C. Jones, 2010: Interactions between nitrogen
deposition, land cover conversion, and climate change determine the
contemporary carbon balance of Europe. Biogeosciences, 7(9), 2749-2764.
Ciais, P., M. Reichstein, N. Viovy, A. Granier, J. Ogee, V. Allard, M. Aubinet, N.
Buchmann, C. Bernhofer, A. Carrara, F. Chevallier, N. De Noblet, A.D. Friend, P.
Friedlingstein, T. Grunwald, B. Heinesch, P. Keronen, A. Knohl, G. Krinner, D.
Loustau, G. Manca, G. Matteucci, F. Miglietta, J.M. Ourcival, D. Papale, K.
Pilegaard, S. Rambal, G. Seufert, J.F. Soussana, M.J. Sanz, E.D. Schulze, T. Vesala,
and R. Valentini, 2005: Europe-wide reduction in primary productivity caused
by the heat and drought in 2003. Nature, 437(7058), 529-533.
Ciais, P., M.J. Schelhaas, S. Zaehle, L. Piao, A. Cescatti, J. Liski, S. Luyssaert, G. Le-Maire,
E.D. Schulze, O. Bouriaud, A. Freibauer, R. Valentini, and G.J. Nabuurs, 2008:
Carbon accumulation in European forests. Nature Geoscience, 1(7), 425-429.
Clark, C.M. and D. Tilman, 2008: Loss of plant species after chronic low-level nitrogen
deposition to prairie grasslands. Nature, 451(7179), 712-715.
Clark, D.A., S.C. Piper, C.D. Keeling, and D.B. Clark, 2003: Tropical rain forest tree
growth and atmospheric carbon dynamics linked to interannual temperature
variation during 1984-2000. Proceedings of the National Academy of Sciences
of the United States of America, 100(10), 5852-5857.
Clark, J.S., 1998: Why trees migrate so fast: confronting theory with dispersal biology
and the paleorecord. American Naturalist, 152(2), 204-224.
Clark, P.U., A.S. Dyke, J.D. Shakun, A.E. Carlson, J. Clark, B. Wohlfarth, J.X. Mitrovica,
S.W. Hostetler, and A.M. McCabe, 2009: The Last Glacial Maximum. Science,
325(5941), 710-714.
Clarke, H., C. Lucas, and P. Smith, 2013: Changes in Australian fire weather between
1973 and 2010. International Journal of Climatology, 33(4), 931-944.
Claussen, M., 2009: Late Quaternary vegetation-climate feedbacks. Climate of the
Past, 5(2), 203-216.
Claussen, M., K. Selent, V. Brovkin, T. Raddatz, and V. Gayler, 2013: Impact of CO
2
and climate on Last Glacial Maximum vegetation – a factor separation.
Biogeosciences, 10(6), 3593-3604.
Clavero, M., D. Villero, and L. Brotons, 2011: Climate change or land use dynamics:
Do we know what climate change indicators indicate? PLoS One, 6(4), e18581,
doi:10.1371/journal.pone.0018581.
Cleland, E. and W.S. Harpole, 2010: Nitrogen enrichment and plant communities.
Annals of the New York Academy of Sciences, 1195(1), 46-61.
Cleland, E.E., I. Chuine, A. Menzel, H.A. Mooney, and M.D. Schwartz, 2007: Shifting
plant phenology in response to global change. Trends in Ecology & Evolution,
22(7), 357-365.
Cleland, E.E., J.M. Allen, T.M. Crimmins, J.A. Dunne, S. Pau, S. Travers, E.S. Zavaleta,
and E.M. Wolkovich, 2012: Phenological tracking enables positive species
responses to climate change. Ecology, 93(8), 1765-1771.
Clements, D.R. and A. Ditommaso, 2011: Climate change and weed adaptation: can
evolution of invasive plants lead to greater range expansion than forecasted?
Weed Research, 51(3), 227-240.
Cobben, M.M.P., J. Verboom, P.F.M. Opdam, R.F. Hoekstra, R. Jochem, and M.J.M.
Smulders, 2012: Wrong place, wrong time: climate change-induced range shift
across fragmented habitat causes maladaptation and declined population size
in a modelled bird species. Global Change Biology, 18(8), 2419-2428.
Cochrane, M.A., 2003: Fire science for rainforests. Nature, 421, 913-919.
Cochrane, M.A. and C.P. Barber, 2009: Climate change, human land use and future
fires in the Amazon. Global Change Biology, 15(3), 601-612.
Cole, J.J., Y.T. Prairie, N.F. Caraco, W.H. McDowell, L.J. Tranvik, R.G. Striegl, C.M. Duarte,
P. Kortelainen, J.A. Downing,J.J. Middelburg, and J. Melack, 2007: Plumbing the
global carbon cycle: integrating inland waters into the terrestrial carbon budget.
Ecosystems, 10(1), 171-184.
Collatz, M.H., M. Ribbas-Carbo, and J.A. Berry, 1992: Coupled photosynthesis –
stomatal conductances model for leaves of C
4
plants. Australian Journal of
Plant Physiology, 19, 519-538.
Collins, J.P., 2010: Amphibian decline and extinction: what we know and what we
need to learn. Diseases of Aquatic Organisms, 92(2-3), 93-99.
Colls, A., N. Ash, and N. Ikkala, 2009: Ecosystem-based Adaptation: A Natural
Response to Climate Change. International Union for Conservation of Nature
and Natural Resources (IUCN), Gland, Switzerland, 16 pp.
Colwell, R.K., G. Brehm, C.L. Cardelus, A.C. Gilman, and J.T. Longino, 2008: Global
warming, elevational range shifts, and lowland biotic attrition in the wet tropics.
Science, 322(5899), 258-261.
Comte, L. and G. Grenouillet, 2013: Do stream fish track climate change? Assessing
distribution shifts in recent decades. Ecography, 36(11), 1236-1246.
Comte, L., L. Buisson, M. Daufresne, and G. Grenouillet, 2013: Climate-induced
changes in the distribution of freshwater fish: observed and predicted trends.
Freshwater Biology, 58(4), 625-639.
Conlisk, E., D. Lawson, A.D. Syphard, J. Franklin, L. Flint, A. Flint, and H.M. Regan, 2012:
The roles of dispersal, fecundity, and predation in the population persistence
of an oak (Quercus engelmannii) under global change. PLoS One, 7(5), e36391,
doi:10.1371/journal.pone.0036391.
Connell, J.H., 1978: Diversity in tropical rain forests and coral reefs. Science,
199(4335), 1302-1310.
Cook, B.I., E.R. Cook, P.C. Huth, J.E. Thompson, A. Forster, and D. Smiley, 2008: A cross-
taxa phenological dataset from Mohonk Lake, NY and its relationship to climate.
International Journal of Climatology, 28(10), 1369-1383.
Cook, B.I., E.M. Wolkovich, T.J. Davies, T.R. Ault, J.L. Betancourt, J.M. Allen, K. Bolmgren,
E.E. Cleland, T.M. Crimmins, N.J.B. Kraft, L.T. Lancaster, S.J. Mazer, G.J. McCabe, B.J.
McGill, C. Parmesan, S. Pau, J. Regetz, N. Salamin, M.D. Schwartz, and S.E. Travers,
2012a: Sensitivity of spring phenology to warming across temporal and spatial
climate gradients in two independent databases. Ecosystems, 15(8), 1283-1294.
Cook, B.I., E.M. Wolkovich, and C. Parmesan, 2012b: Divergent responses to spring
and winter warming drive community level flowering trends. Proceedings of
the National Academy of Sciences of the United States of America, 109(23),
9000-9005.
Cooper, O.R., D.D. Parrish, A. Stohl, M. Trainer, P. Nedelec, V. Thouret, J.P. Cammas, S.J.
Oltmans, B.J. Johnson, D. Tarasick, T. Leblanc, I.S. McDermid, D. Jaffe, R. Gao, S.
Stith, T. Ryerson, K. Aikin, T. Campos, A. Weinheimer, and A.M. Avery, 2010:
Increasing springtime ozone mixing ratios in the free troposphere over western
North America. Nature, 463(12), 344-348.
Corlett, R.T., 2011: Impacts of warming on tropical lowland rainforests. Trends in
Ecology & Evolution, 26(11), 606-613.
Costa, M.H., S.N.M. Yanagi, P. Souza, A. Ribeiro, and E.J.P. Rocha, 2007: Climate
change in Amazonia caused by soybean cropland expansion, as compared to
caused by pastureland expansion. Geophysical Research Letters, 34(7), L07706,
doi:10.1029/2007GL029271.
Cox, P.M., C. Huntingford, and R.J. Harding, 1998: A canopy conductance and
photosynthesis model for use in a GCM land surface scheme. Journal of
Hydrology, 212-213, 79-94.
Cox, P.M., D. Pearson, B.B. Booth, P. Friedlingstein, C. Huntingford, C.D. Jones, and
C.M. Luke, 2013: Sensitivity of tropical carbon to climate change constrained
by carbon dioxide variability. Nature, 494(7437), 341-344.
Crimmins, S.M., S.Z. Dobrowski, J.A. Greenberg, J.T. Abatzoglou, and A.R. Mynsberge,
2011: Changes in climatic water balance drive downhill shifts in plant species’
optimum elevations. Science, 331(6015), 324-327.
Crimmins, T.M., M.A. Crimmins, and C.D. Bertelsen, 2009: Flowering range changes
across an elevation gradient in response to warming summer temperatures.
Global Change Biology, 15(5), 1141-1152.
Crossman, J., M.N. Futter, S.K. Oni, P.G. Whitehead, L. Jin, D. Butterfield, H.M. Baulch,
and P.J. Dillon, 2013: Impacts of climate change on hydrology and water quality:
future proofing management strategies in the Lake Simcoe watershed, Canada.
Journal of Great Lakes Research, 39(1), 19-32.
Crous, C.J., S.M. Jacobs, and K.J. Esler, 2012: Drought-tolerance of an invasive alien
tree, Acacia mearnsii and two native competitors in fynbos riparian ecotones.
Biological Invasions, 14(3), 619-631.
Cui, X.F. and H.F. Graf, 2009: Recent land cover changes on the Tibetan Plateau: a
review. Climatic Change, 94(1-2), 47-61.
Curran, L.M., S.N. Trigg, A.K. McDonald, D. Astiani, Y.M. Hardiono, P. Siregar, I. Caniago,
and E. Kasischke, 2004: Lowland forest loss in protected areas of Indonesian
Borneo. Science, 303(5660), 1000-1003.
da Costa, A.C.L., D. Galbraith, S. Almeida, B.T.T. Portela, M. Da Costa, J. De Athaydes
Silva Junior, A.P. Braga, P.H.L. De Gonçalves, A.A. De Oliveira, R. Fisher, O.L.
Phillips, D.B. Metcalfe, P. Levy, and P. Meir, 2010: Effect of 7 yr of experimental
drought on vegetation dynamics and biomass storage of an eastern Amazonian
rainforest. New Phytologist, 187(3), 579-591.
Dahm, C.N., M.A. Baker, D.I. Moore, and J.R. Thibault, 2003: Coupled biogeochemical
and hydrological responses of streams and rivers to drought. Freshwater
Biology, 48(7), 1219-1231.

335
Terrestrial and Inland Water Systems Chapter 4
4
Dai, F., Z. Su, S. Liu, and G. Liu, 2011: Temporal variation of soil organic matter content
and potential determinants in Tibet, China. Catena, 85(3), 288-294.
Dale, V.H., M.L. Tharp, K.O. Lannom, and D.G. Hodges, 2010: Modeling transient
response of forests to climate change. Science of the Total Environment, 408(8),
1888-1901.
Danby, R.K., and D.S. Hik, 2007: Variability, contingency and rapid change in recent
subarctic alpine tree line dynamics. Journal of Ecology, 95(2), 352-363.
Daniau, A.L., S.P. Harrison, and P.J. Bartlein, 2010: Fire regimes during the Last Glacial.
Quaternary Science Reviews, 29(21-22), 2918-2930.
Daniau, A.L., P.J. Bartlein, S.P. Harrison, I.C. Prentice, S. Brewer, P. Friedlingstein, T.I.
Harrison-Prentice, J. Inoue, K. Izumi, J.R. Marlon, S. Mooney, M.J. Power, J.
Stevenson, W. Tinner, M. Andric, J. Atanassova, H. Behling, M. Black, O. Blarquez,
K.J. Brown, C. Carcaillet, E.A. Colhoun, D. Colombaroli, B.A.S. Davis, D. D’Costa,
J. Dodson, L. Dupont, Z. Eshetu, D.G. Gavin, A. Genries, S. Haberle, D.J. Hallett, G.
Hope, S.P. Horn, T.G. Kassa, F. Katamura, L.M. Kennedy, P. Kershaw, S. Krivonogov,
C. Long, D. Magri, E. Marinova, G.M. McKenzie, P.I. Moreno, P. Moss, F.H.
Neumann, E. Norstrom, C. Paitre, D. Rius, N. Roberts, G.S. Robinson, N. Sasaki,
L. Scott, H. Takahara, V. Terwilliger, F. Thevenon, R. Turner, V.G. Valsecchi, B.
Vanniere, M. Walsh, N. Williams, and Y. Zhang, 2012: Predictability of biomass
burning in response to climate changes. Global Biogeochemical Cycles, 26(4),
GB4007, doi:10.1029/2011GB004249.
Daufresne, M. and P. Boet, 2007: Climate change impacts on structure and diversity
of fish communities in rivers. Global Change Biology, 13(12), 2467-2478.
Davidson, A.M., M. Jennions, and A.B. Nicotra, 2011: Do invasive species show higher
phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis.
Ecology Letters, 14(4), 419-431.
Davidson, E.A. and I.A. Janssens, 2006: Temperature sensitivity of soil carbon
decomposition and feedbacks to climate change. Nature, 440, 165-173.
Davidson, E.A., A.C. de Araujo, P. Artaxo, J.K. Balch, I.F. Brown, M.M.C. Bustamante,
M.T. Coe, R.S. DeFries, M. Keller, M. Longo, J.W. Munger, W. Schroeder, B.S.
Soares, C.M. Souza, and S.C. Wofsy, 2012: The Amazon basin in transition.
Nature, 481(7381), 321-328.
Davies, P.M., 2010: Climate change implications for river restoration in global
biodiversity hotspots. Restoration Ecology, 18(3), 261-268.
Davin, E.L. and N. de Noblet-Ducoudre, 2010: Climatic impact of global-scale
deforestation: radiative versus nonradiative processes. Journal of Climate,
23(1), 97-112.
Davin, E.L., N. de Noblet-Ducoudre, and P. Friedlingstein, 2007: Impact of land cover
change on surface climate: relevance of the radiative forcing concept.
Geophysical Research Letters, 34(13), L13702, doi:10.1029/2007GL029678.
Davis, C.C., C.G. Willis, R.B. Primack, and A.J. Miller-Rushing, 2010: The importance
of phylogeny to the study of phenological response to global climate change.
Philosophical Transactions of the Royal Society B, 365(1555), 3201-3213.
Davis, J.L., S. Lake, and R. Thompson, 2010: Freshwater biodiversity and climate
change. In: Managing Climate Change: Papers from the Greenhouse 2009
Conference [Jubb, I., P. Holper, and W. Cai (eds.)]. Commonwealth Scientific and
Industrial Research Organisation (CSIRO) Publishing, Collingwood, Australia,
pp. 73-84.
Dawes, M.A., S. Hättenschwiler, P. Bebi, F. Hagedorn, I.T. Handa, C. Körner, and
C. Rixen, 2011: Species-specific tree growth responses to 9years of CO
2
enrichment at the alpine treeline. Journal of Ecology, 99(2), 383-394.
Dawson, T.P., S.T. Jackson, J.I. House, I.C. Prentice, and G.M. Mace, 2011: Beyond
predictions: biodiversity conservation in a changing climate. Science,
332(6025), 53-58.
de Jong, R., S. de Bruin, A. de Wit, M.E. Schaepman, and D.L. Dent, 2011: Analysis of
monotonic greening and browning trends from global NDVI time-series.
Remote Sensing of Environment, 115(2), 692-702.
De Kauwe, M.G., B.E. Medlyn, S. Zaehle, A.P. Walker, M.C. Dietze, T. Hickler, A.K. Jain,
Y. Luo, W.J. Parton, C. Prentice, B. Smith, P.E. Thornton, S. Wang, Y.-P. Wang, D.
Wårlind, E.S. Weng, K.Y. Crous, D.S. Ellsworth, P.J. Hanson, H. Seok-Kim, J.M.
Warren, R. Oren, and R.J. Norby, 2013: Forest water use and water use efficiency
at elevated CO
2
: a model-data intercomparison at two contrasting temperate
forest FACE sites. Global Change Biology, 19(6), 1759-1779.
De Michele, C., F. Accatino, R. Vezzoli, and R.J. Scholes, 2011: Savanna domain in
the herbivores-fire parameter space exploiting a tree–grass–soil water dynamic
model. Journal of Theoretical Biology, 289, 74-82.
de Noblet-Ducoudre, N., J.P. Boisier, A. Pitman, G.B. Bonan, V. Brovkin, F. Cruz, C.
Delire, V. Gayler, B.J.J.M. van den Hurk, P.J. Lawrence, M.K. van der Molen, C.
Muller, C.H. Reick, B.J. Strengers, and A. Voldoire, 2012: Determining robust
impacts of land-use-induced land cover changes on surface climate over North
America and Eurasia: results from the first set of LUCID experiments. Journal
of Climate, 25(9), 3261-3281.
de Torres Curth, M.I., L. Ghermandi, and C. Biscayart, 2012: Are Fabiana imbricata
shrublands advancing over northwestern Patagonian grasslands? A population
dynamics study involving fire and precipitation. Journal of Arid Environments,
83, 78-85.
de Vries, W. and M. Posch, 2011: Modelling the impact of nitrogen deposition,
climate change and nutrient limitations on tree carbon sequestration in Europe
for the period 1900-2050. Environmental Pollution, 159(10), 2289-2299.
DeFries, R.S., T. Rudel, M. Uriarte, and M. Hansen, 2010: Deforestation driven by
urban population growth and agricultural trade in the twenty-first century.
Nature Geoscience, 3(3), 178-181.
Delire, C., N. de Noblet-Ducoudre, A. Sima, and I. Gouirand, 2011: Vegetation
dynamics enhancing long-term climate variability confirmed by two models.
Journal of Climate, 24(9), 2238-2257.
Denman, K.L., G. Brasseur, A. Chidthaisong, P. Ciais, P.M. Cox, R.E. Dickinson, D.
Hauglustaine, C. Heinze, E. Holland, D. Jacob, U. Lohmann, S. Ramachandran,
P.L. da Silva Dias, S.C. Wofsy, and X. Zhang, 2007: Couplings between changes in
the climate system and biogeochemistry. In: Climate Change 2007: The Physical
Science Basis. Contribution of Working Group I to the Fourth Assessment Report
of the Intergovernmental Panel on Climate Change [Solomon, S., D. Qin, M.
Manning, Z. Chen, M. Marquis, K. B. Averyt, M. Tignor, and H. L. Miller (eds.)].
Cambridge University Press, Cambridge, UK and New York, NY, USA., pp. 499-587.
Dentener, F., T. Keating, and H. Akimoto (eds.), 2010: Hemispheric Transport of Air
Pollution 2010: Part A – Ozone and Particulate Matter. United Nations Economic
Commission for Europe (UNECE) Air Pollution Series Studies No. 17, United
Nations, New York, NY, USA and Geneva, Switzerland, 304 pp.
DeRose, R.J. and J.N. Long, 2012: Drought-driven disturbance history characterizes
a southern Rocky Mountain subalpine forest. Canadian Journal of Forest
Research / Revue Canadienne De Recherche Forestiere, 42(9), 1649-1660.
Deutsch, C.A., J.J. Tewksbury, R.B. Huey, K.S. Sheldon, C.K. Ghalambor, D.C. Haak,
and P.R. Martin, 2008: Impacts of climate warming on terrestrial ectotherms
across latitude. Proceedings of the National Academy of Sciences of the United
States of America, 105(18), 6668-6672.
Devi, N., F. Hagedorn, P. Moiseev, H. Bugmann, S. Shiyatov, V. Mazepa, and A. Rigling,
2008: Expanding forests and changing growth forms of Siberian larch at the Polar
Urals treeline during the 20
th
century. Global Change Biology, 14(7), 1581-1591.
Devictor, V., C. van Swaay, T. Brereton, L. Brotons, D. Chamberlain, J. Heliola, S.
Herrando, R. Julliard, M. Kuussaari, A. Lindstrom, J. Reif, D.B. Roy, O. Schweiger,
J. Settele, C. Stefanescu, A. Van Strien, C. Van Turnhout, Z. Vermouzek, M. Wallis
DeVries, I. Wynhoff, and F. Jiguet, 2012: Differences in the climatic debts of birds
and butterflies at a continental scale. Nature Climate Change, 2(2), 121-124.
Dial, R.J., E.E. Berg, K. Timm, A. McMahon, and J. Geck, 2007: Changes in the alpine
forest-tundra ecotone commensurate with recent warming in southcentral
Alaska: evidence from orthophotos and field plots. Journal of Geophysical
Research: Biogeosciences, 112(G4), G04015, doi:10.1029/2007JG000453.
Dieleman, W.I.J., S. Vicca, F.A. Dijkstra, F. Hagedorn, M.J. Hovenden, K.S. Larsen, J.A.
Morgan, A. Volder, C. Beier, J.S. Dukes, J. King, S. Leuzinger, S. Linder, Y. Luo, R.
Oren, P. De Angelis, D. Tingey, M.R. Hoosbeek, and I.A. Janssens, 2012: Simple
additive effects are rare: a quantitative review of plant biomass and soil process
responses to combined manipulations of CO
2
and temperature. Global Change
Biology, 18(9), 2681-2693.
Diez, J.M., C.M. D’Antonio, J.S. Dukes, E.D. Grosholz, J.D. Olden, C.J.B. Sorte, D.M.
Blumenthal, B.A. Bradley, R. Early, I. Ibanez, S.J. Jones, J.J. Lawler, and L.P. Miller,
2012: Will extreme climatic events facilitate biological invasions? Frontiers in
Ecology and the Environment, 10(5), 249-257.
Diffenbaugh, N.S. and F. Giorgi, 2012: Climate change hotspots in the CMIP5 global
climate model ensemble. Climatic Change, 114(3-4), 813-822.
Diffenbaugh, N.S., J.S. Pal, R.J. Trapp, and F. Giorgi, 2005: Fine-scale processes regulate
the response of extreme events to global climate change. Proceedings of the
National Academy of Sciences of the United States of America, 102(44), 15774-
15778.
Dise, N.B., 2009: Peatland response to global change. Science, 326(5954), 810-811.
Doak, D.F. and W.F. Morris, 2010: Demographic compensation and tipping points in
climate-induced range shifts. Nature, 467(7318), 959-962.
Dobrowski, S.Z., J. Abatzoglou, A.K. Swanson, J.A. Greenberg, A.R. Mynsberge, Z.A.
Holden, and M.K. Schwartz, 2013: The climate velocity of the contiguous United
States during the 20
th
century. Global Change Biology, 19(1), 241-251.

336
Chapter 4 Terrestrial and Inland Water Systems
4
Dohrenbusch, A. and A. Bolte, 2007: Forest plantations. In: Wood Production, Wood
Technology and Biotechnological Impacts [Kües, U. (ed.)]. Universitätsverlag
Göttingen, Göttingen, Germany, pp. 73-83.
Donnelly, A., A. Caffarra, C.T. Kelleher, B.F. O’Neill, E. Diskin, A. Pletsers, H. Proctor, R.
Stirnemann, J. O’Halloran, J. Penuelas, T.R. Hodkinson, and T.H. Sparks, 2012:
Surviving in a warmer world: environmental and genetic responses. Climate
Research, 53(3), 245-262.
Donohue, R.J., T.R. McVicar, M.L. Roderick,, and G.D. Farquhar, 2013: Impact of CO
2
fertilization on maximum foliage coveracross the globe´s warm, arid environments.
Geophysical Research Letters, 40, 3031-3035.
Doughty, C.E. and M.L. Goulden, 2008: Are tropical forests near a high temperature
threshold? Journal of Geophysical Research: Biogeosciences, 113(G1), G00B07,
doi:10.1029/2007JG000632.
Doughty, C.E., C.B. Field, and A.M.S. McMillan, 2011: Can crop albedo be increased
through modification of leaf trichomes, and could this cool the regional climate?
Climatic Change, 104(2), 379-387.
Douville, H., A. Ribes, B. Decharme, R. Alkama, and J. Sheffield, 2013: Anthropogenic
influence on multidecadal changes in reconstructed global evapotranspiration.
Nature Climate Change, 3(1), 59-62.
Doxford, S.W. and R.P. Freckleton, 2012: Changes in the large-scale distribution of
plants: extinction, colonisation and the effects of climate. Journal of Ecology,
100(2), 519-529.
Drewitt, A.L. and R.H.W. Langston, 2006: Assessing the impacts of wind farms on
birds. Ibis, 148, 29-42.
Dudgeon, D., A.H. Arthington, M.O. Gessner, Z.I. Kawabata, D.J. Knowler, C. Leveque,
R.J. Naiman, A.H. Prieur-Richard, D. Soto, M.L.J. Stiassny, and C.A. Sullivan, 2006:
Freshwater biodiversity: importance, threats, status and conservation challenges.
Biological Reviews, 81(2), 163-182.
Dukes, J.S., J. Pontius, D. Orwig, J.R. Garnas, V.L. Rodgers, N. Brazee, B. Cooke, K.A.
Theoharides, E.E. Stange, R. Harrington, J. Ehrenfeld, J. Gurevitch, M. Lerdau, K.
Stinson, R. Wick, and M. Ayres, 2009: Responses of insect pests, pathogens, and
invasive plant species to climate change in the forests of northeastern North
America: what can we predict? Canadian Journal of Forest Research / Revue
Canadienne De Recherche Forestiere, 39(2), 231-248.
Dulamsuren, C., M. Hauck, S. Nyambayar, M. Bader, D. Osokhjargal, S. Oyungerel,
and C. Leuschner, 2009: Performance of Siberian elm (Ulmus pumila) on steppe
slopes of the northern Mongolian mountain taiga: drought stress and herbivory
in mature trees. Environmental and Experimental Botany, 66(1), 18-24.
Dullinger, S., A. Gattringer, W. Thuiller, D. Moser, N.E. Zimmermann, A. Guisan, W.
Willner, C. Plutzar, M. Leitner, T. Mang, M. Caccianiga, T. Dirnbock, S. Ertl, A.
ischer, J. Lenoir, J.C. Svenning, A. Psomas, D.R. Schmatz, U. Silc, P. Vittoz, and K.
Hulber, 2012: Extinction debt of high-mountain plants under twenty-first-
century climate change. Nature Climate Change, 2(8), 619-622.
Dunlop, M., D.W. Hilbert, S. Ferrier, A. House, A. Liedloff, S.M. Prober, A. Smyth, T.G.
Martin, T. Harwood, K.J. Williams, C. Fletcher, and H. Murphy, 2012: The
Implications of Climate Change for Biodiversity Conservation and the National
Reserve System: Final Synthesis. Canberra, ACT, Australia, 80 pp.
Dunn, R.R., N.C. Harris, R.K. Colwell, L.P. Koh, and N.S. Sodhi, 2009: The sixth mass
coextinction: are most endangered species parasites and mutualists? Proceedings
of the Royal Society B, 276(1670), 3037-3045.
Durance, I. and S.J. Ormerod, 2007: Climate change effects on upland stream
macroinvertebrates over a 25-year period. Global Change Biology, 13(5), 942-
957.
Eamus, D. and A.R. Palmer, 2007: Is climate change a possible explanation for woody
thickening in arid and semi-arid regions? International Journal of Ecology,
2007, 37364, doi:10.1155/2007/37364.
Eastaugh, C.S., E. Potzelsberger, and H. Hasenauer, 2011: Assessing the impacts of
climate change and nitrogen deposition on Norway spruce (Picea abies L. Karst)
growth in Austria with BIOME-BGC. Tree Physiology, 31(3), 262-274.
Edburg, S.L., J.A. Hicke, P.D. Brooks, E.G. Pendall, B.E. Ewers, U. Norton, D. Gochis,
E.D. Gutmann, and A.J.H. Meddens, 2012: Cascading impacts of bark beetle-
caused tree mortality on coupled biogeophysical and biogeochemical processes.
Frontiers in Ecology and the Environment, 10(8), 416-424.
Eggermont, H., D. Verschuren, L. Audenaert, L. Lens, J. Russell, G. Klaassen, and O.
Heiri, 2010: Limnological and ecological sensitivity of Rwenzori mountain lakes
to climate warming. Hydrobiologia, 648(1), 123-142.
Eisenhauer, N., S. Cesarz, R. Koller, K. Worm, and P.B. Reich, 2012: Global change
belowground: impacts of elevated CO
2
, nitrogen, and summer drought on soil
food webs and biodiversity. Global Change Biology, 18(2), 435-447.
Eliasch, J., 2008: Climate Change: Financing Global Forests – The Eliasch Review.
Earthscan, Abingdon, UK and New York, NY, USA , 288 pp.
Elith, J. and J.R. Leathwick, 2009: Species distribution models: ecological explanation
and prediction across space and time. Annual Review of Ecology Evolution and
Systematics, 40, 677-697.
Ellery, W.N., R.J. Scholes, and M.T. Mentis, 1991: An initial approach to predicting
the sensitivity of the South African grassland biome to climate change. South
African Journal of Science, 87, 499-503.
Elmendorf, S.C., G.H.R. Henry, R.D. Hollister, R.G. Bjork, N. Boulanger-Lapointe, E.J.
Cooper, J.H.C. Cornelissen, T.A. Day, E. Dorrepaal, T.G. Elumeeva, M. Gill, W.A.
Gould, J. Harte, D.S. Hik, A. Hofgaard, D.R. Johnson, J.F. Johnstone, I.S. Jonsdottir,
J.C. Jorgenson, K. Klanderud, J.A. Klein, S. Koh, G. Kudo, M. Lara, E. Levesque,
B. Magnusson, J.L. May, J.A. Mercado-Diaz, A. Michelsen, U. Molau, I.H. Myers-
Smith, S.F. Oberbauer, V.G. Onipchenko, C. Rixen, N. Martin Schmidt, G.R. Shaver,
M.J. Spasojevic, o.E. orhallsdottir, A. Tolvanen, T. Troxler, C.E. Tweedie, S. Villareal,
C.-H. Wahren, X. Walker, P.J. Webber, J.M. Welker, and S. Wipf, 2012: Plot-scale
evidence of tundra vegetation change and links to recent summer warming.
Nature Climate Change, 2(6), 453-457.
Elser, J.J., M.E.S. Bracken, E.E. Cleland, D.S. Gruner, W.S. Harpole, H. Hillebrand, J.T.
Ngai, E.W. Seabloom, J.B. Shurin, and J.E. Smith, 2007: Global analysis of nitrogen
and phosphorus limitation of primary producers in freshwater, marine and
terrestrial ecosystems. Ecology Letters, 10, 1135-1142.
Elser, J.J., T. Andersen, J.S. Baron, A.K. Bergström, M. Jansson, M. Kyle, K.R. Nydick, L.
Steger, and D.O. Hessen, 2009: Shifts in lake N:P stoichiometry and nutrient
limitation driven by atmospheric nitrogen deposition. Science, 326(5954), 835-
837.
Emmett, B.A., C. Beier, M. Estiarte, A. Tietema, H.L. Kristensen, D. Williams, J. Penuelas,
I. Schmidt, and A. Sowerby, 2004: The response of soil processes to climate change:
results from manipulation studies of shrublands across an environmental
gradient. Ecosystems, 7(6), 625-637.
Engler, R., C.F. Randin, W. Thuiller, S. Dullinger, N.E. Simmermann, M.B. Araujo, P.B.
Pearman, G. Le Lay, C. Peidallu, C.H. Albert, P. Choler, G. Coldea, S. De Lamo, T.
Dirnbock, J.C. Gegout, D. Gomez-Garcia, J.A. Grytnes, E. Heegaard, F. Hoistad,
D. Nogues-Bravo, S. Normand, M. Puscas, M.T. Sebastia, A. Stanisci, J.P. Theurillat,
M.R. Trivedi, P. Vittoz, and A. Guisan, 2011: 21
st
century climate change threatens
mountain flora unequally across Europe. Global Change Biology, 17(7), 2330-
2341.
Enquist, B.J. and C.A.F. Enquist, 2011: Long-term change within a Neotropical forest:
assessing differential functional and floristic responses to disturbance and
drought. Global Change Biology, 17(3), 1408-1424.
Epstein, H., J. Kaplan, H. Lischke, and Q. Yu, 2007: Simulating future changes in arctic
tundra and sub-arctic vegetation. Computing in Science and Engineering, 9,
12-23.
Epstein, H.E., D. A. Walker, M. K. Raynolds, G. J. Jia, and A. M. Kelley, 2008: Phytomass
patterns across a temperature gradient of the North American arctic tundra.
Journal of Geophysical Research: Biogeosciences, 113(G3), G03S02, doi:10.1029/
2007JG000555.
Erlandsson, M., I. Buffam, J. Folster, H. Laudon, J. Temnerud, G.A. Weyhenmeyer,
and K. Bishop, 2008: Thirty-five years of synchrony in the organic matter
concentrations of Swedish rivers explained by variation in flow and sulphate.
Global Change Biology, 14(5), 1191-1198.
Erskine, P.D., D. Lamb, and M. Bristow, 2006: Tree species diversity and ecosystem
function: can tropical multi-species plantations generate greater productivity?
Forest Ecology and Management, 233(2-3), 205-210.
Essl, F., S. Dullinger, D. Moser, W. Rabitsch, and I. Kleinbauer, 2012: Vulnerability of
mires under climate change: implications for nature conservation and climate
change adaptation. Biodiversity and Conservation, 21(3), 655-669.
EU Council, 1992: Council Directive 92/43/EEC of 21 May 1992 on the Conservation
of Natural Habitats and of Wild Fauna and Flora. The Council of the European
Communities, Brussels, Belgium, 66 pp.
Euskirchen, E.S., A.D. McGuire, F.S. Chapin III, S. Yi, and C.C. Thompson, 2009: Changes
in vegetation in northern Alaska under scenarios of climate change, 2003-2100:
implications for climate feedbacks. Ecological Applications, 19(4), 1022-1043.
Evans, C.D., D.T. Monteith, and D.M. Cooper, 2005: Long-term increases in surface
water dissolved organic carbon: observations, possible causes and environmental
impacts. Environmental Pollution, 137(1), 55- 71.
Eycott, A.E., G.B. Stewart, L.M. Buyung-Ali, D.E. Bowler, K. Watts, and A.S. Pullin,
2012: A meta-analysis on the impact of different matrix structures on species
movement rates. Landscape Ecology, 27(9), 1263-1278.

337
Terrestrial and Inland Water Systems Chapter 4
4
Fahey, T.J., 1998: Recent changes in an upland forest in South-Central New York.
Journal of the Torrey Botanical Society, 125(1), 51-59.
Fall, S., D. Niyogi, A. Gluhovsky, R.A. Pielke, E. Kalnay, and G. Rochon, 2010: Impacts
of land use land cover on temperature trends over the continental United
States: assessment using the North American Regional Reanalysis. International
Journal of Climatology, 30(13), 1980-1993.
Falloon, P.D., R. Dankers, R.A. Betts, C.D. Jones, B.B.B. Booth, and F.H. Lambert, 2012:
Role of vegetation change in future climate under the A1B scenario and a
climate stabilisation scenario, using the HadCM3C earth systems model.
Biogeosciences, 9(11), 4739-4756.
FAO, 2005: Global Forest Resources Assessment 2005. FAO Forestry Paper No. 147,
Food and Agriculture Organization of the United Nations (FAO), Rome, Italy,
350 pp.
FAO, 2010: Global Forest Resources Assessment 2010. FAO Forestry Paper No. 163,
Food and Agriculture Organization of the United Nations (FAO), Rome, Italy,
340 pp.
Farquhar, G.D., S. von Caemmerer, and J.A. Berry, 1980: A biochemical model of
photosynthetic CO
2
assimilation in leaves of C3 species. Planta, 149, 78-90.
Fauset, S., T.R. Baker, S.L. Lewis, T.R. Feldpausch, K. Affum-Baffoe, E.G. Foli, K.C. Hamer,
and M.D. Swaine, 2012: Drought-induced shifts in the floristic and functional
composition of tropical forests in Ghana. Ecology Letters, 15(10), 1120-1129.
Fay, P.A., J.D. Carlisle, A.K. Knapp, J.M. Blair, and S.L. Collins, 2003: Productivity
responses to altered rainfall patterns in a C
4
-dominated grassland. Oecologia,
137(2), 245-251.
Feeley, K.J. and E.M. Rehm, 2012: Amazon’s vulnerability to climate change heightened
by deforestation and man-made dispersal barriers. Global Change Biology,
18(12), 3606-3614.
Fellows, A.W. and M.L. Goulden, 2012: Rapid vegetation redistribution in Southern
California during the early 2000s drought. Journal of Geophysical Research:
Biogeosciences, 117(G3), G03025, doi:10.1029/2012JG002044.
Fensham, R.J., R.J. Fairfax, and D.P. Ward, 2009:Drought-induced tree death in
savanna. Global Change Biology, 15, 380-387.
Fensham, R.J., R.J. Fairfax, and J.M. Dwyer, 2012: Potential aboveground biomass in
drought-prone forest used for rangeland pastoralism. Ecological Applications,
22(3), 894-908.
Fensholt, R., T. Langanke, K. Rasmussen, A. Reenberg, S.D. Prince, C. Tucker, B. Scholes,
Q.B. Le, A. Bondeau, R. Eastman, H. Epstein, A.E. Gaughan, U. Hellden, C. Mbow,
L. Olsson, J. Paruelo, C. Schweitzer, J. Seaquist, and K. Wessels, 2012: Greenness
in semi-arid areas across the globe 1981-2007 – an Earth Observing Satellite
based analysis of trends and drivers. Remote Sensing of Environment, 121,
144-158.
Ferriere, R. and S. Legendre, 2013: Eco-evolutionary feedbacks, adaptive dynamics
and evolutionary rescue theory. Philosophical Transactions of the Royal Society
B, 368(1610), 20120081, doi:10.1098/rstb.2012.0404.
Ficke, A.D., C.A. Myrick, and L.J. Hansen, 2007: Potential impacts of global climate
change on freshwater fisheries. Reviews in Fish Biology and Fisheries, 17(4),
581-613.
Field, C.B., D.B. Lobell, H.A. Peters, and N.R. Chiariello, 2007: Feedbacks of terrestrial
ecosystems to climate change. Annual Review of Environment and Resources,
32, 1-29, doi:10.1146/annurev.energy.32.053006.141119.
Findell, K.L., E. Shevliakova, P.C.D. Milly, and R.J. Stouffer, 2007: Modeled impact of
anthropogenic land cover change on climate. Journal of Climate, 20(14), 3621-
3634.
Finn, D.S., K. Khamis, and A.M. Milner, 2013: Loss of small glaciers will diminish beta
diversity in Pyrenean streams at two levels of biological organization. Global
Ecology and Biogeography, 22(1), 40-51.
Finzi, A.C., R.J. Norby, C. Calfapietra, A. Gallet-Budynek, B. Gielen, W.E. Holmes, M.R.
Hoosbeek, C.M. Iversen, R.B. Jackson, M.E. Kubiske, J. Ledford, M. Liberloo, R.
Oren, A. Polle, S. Pritchard, D.R. Zak, W.H. Schlesinger, and R. Ceulemans, 2007:
Increases in nitrogen uptake rather than nitrogen-use efficiency support higher
rates of temperate forest productivity under elevated CO
2
. Proceedings of the
National Academy of Sciences of the United States of America, 104(35), 14014-
14019.
Fiorese, G. and G. Guariso, 2013: Modeling the role of forests in a regional carbon
mitigation plan. Renewable Energy, 52, 175-182.
Fischer, J. and D.B. Lindenmayer, 2007: Landscape modification and habitat
fragmentation: a synthesis. Global Ecology and Biogeography, 16(3), 265-280.
Fischlin, A., G.F. Midgley, J.T. Price, R. Leemans, B. Gopal, C. Turley, M.D.A. Rounsevell,
O.P. Dube, J. Tarazona, and A.A. Velichko, 2007: Ecosystems, their properties,
goods, and services. In: Climate Change 2007: Impacts, Adaptation and
Vulnerability. Contribution of Working Group II to the Fourth Assessment Report
of the Intergovernmental Panel on Climate Change (IPCC) [Parry, M.L., O.F.
Canziani, J.P. Palutikof, P.J. van der Linden, and C.E. Hanson (eds.)]. Cambridge
University Press, Cambridge, UK and New York, NY, USA, pp. 211-272.
Fisher, J.B., G. Hurtt, R.Q. Thomas, and J.Q. Chambers, 2008: Clustered disturbances
lead to bias in large-scale estimates based on forest sample plots. Ecology
Letters, 11(6), 554-563.
Fisher, R., N. McDowell, D. Purves, P. Moorcroft, S. Sitch, P. Cox, C. Huntingford, P. Meir,
and F. Ian Woodward, 2010: Assessing uncertainties in a second-generation
dynamic vegetation model caused by ecological scale limitations. New
Phytologist, 187(3), 666-681.
FLUXNET, 2012: Historical Site Status. fluxnet.ornl.gov/site_status.
Foden, W.B., S.H.M. Butchart, S.N. Stuart, J.C. Vie, H.R. Akcakaya, A. Angulo, L.M.
DeVantier, A. Gutsche, E. Turak, L. Cao, S.D. Donner, V. Katariya, R. Bernard, R.A.
Holland, A.F. Hughes, S.E. O’Hanlon, S.T. Garnett, C.H. Sekercioglu, and G.M.
Mace, 2013: Identifying the world’s most climate change vulnerable species: a
systematic trait-based assessment of all birds, amphibians and corals. PLoS
One, 8(6), e65427, doi:10.1371/journal.pone.0065427.
Folke, C., S. Carpenter, B. Walker, M. Scheffer, T. Elmqvist, L. Gunderson, and C.S. Holling,
2004: Regime shifts, resilience, and biodiversity in ecosystem management.
Annual Review of Ecology Evolution and Systematics, 35, 557-581.
Forbes, B.C., M.M. Fauria, and P. Zetterberg, 2010: Russian Arctic warming and
‘greening’ are closely tracked by tundra shrub willows. Global Change Biology,
16(5), 1542-1554.
Fordham, D.A., H.R. Akcakaya, M.B. Araujo, J. Elith, D.A. Keith, R. Pearson, T.D. Auld,
C. Mellin, J.W. Morgan, T.J. Regan, M. Tozer, M.J. Watts, M. White, B.A. Wintle,
C. Yates, and B.W. Brook, 2012: Plant extinction risk under climate change: are
forecast range shifts alone a good indicator of species vulnerability to global
warming? Global Change Biology, 18(4), 1357-1371.
Fowler, D., M. Coyle, U. Skiba, M.A. Sutton, J.N. Cape, S. Reis, L.J. Sheppard, A. Jenkins,
B. Grizzetti, J.N. Galloway, P. Vitousek, A. Leach, A.F. Bouwman, K. Butterbach-
Bahl, F. Dentener, D. Stevenson, M. Amann, and M. Voss, 2013: The global
nitrogen cycle in the twenty-first century. Philosophical Transactions of the
Royal Society B, 368(1621), 20130164, doi:10.1098/rstb.2013.0164.
Franklin, J., F.W. Davis, M. Ikegami, A.D. Syphard, L.E. Flint, A.L. Flint, and L. Hannah,
2013: Modeling plant species distributions under future climates: how fine
scale do climate projections need to be? Global Change Biology, 19(2), 473-
483.
Franks, S.J. and A.A. Hoffmann, 2012: Genetics of climate change adaptation. Annual
Review of Genetics, 46, 185-208.
Franks, S.J. and A.E. Weis, 2008: A change in climate causes rapid evolution of
multiple life-history traits and their interactions in an annual plant. Journal of
Evolutionary Biology, 21(5), 1321-1334.
Frelich, L.E., R.O. Peterson, M. Dovciak, P.B. Reich, J.A. Vucetich, and N. Eisenhauer,
2012: Trophic cascades, invasive species and body-size hierarchies interactively
modulate climate change responses of ecotonal temperate-boreal forest.
Philosophical Transactions of the Royal Society B, 367(1605), 2955-2961.
Friend, A., W. Lucht, T.T. Rademacher, R.M. Keribin, R. Betts, P. Cadule, P. Ciais, D.B.
Clark, R. Dankers, P. Falloon, A. Ito, R. Kahana, A. Kleidon, M.R. Lomas, K.
Nishina, S. Ostberg, R. Pavlick, P. Peylin, S. Schaphoff, N. Vuichard, L. Warszawski,
A. Wiltshire, and F.I. Woodward, 2013: Carbon residence time dominates
uncertainty in terrestrial vegetation responses to future climate and atmospheric
CO
2
. Proceedings of the National Academy of Science of the United States of
America (in press), doi:10.1073/pnas.1222477110.
Frolking, S., J. Talbot, M.C. Jones, C.C. Treat, J.B. Kauffman, E.S. Tuittila, and N. Roulet,
2011: Peatlands in the Earth’s 21st century climate system. Environmental
Reviews, 19, 371-396.
Fujino, J., R. Nair, M. Kainuma, T. Masui, and Y. Matsuoka, 2006: Multi-gas mitigation
analysis on stabilization scenerios using AIM global model. The Energy Journal,
SI 3, 343-354.
Gagen, M., W. Finsinger, F. Wagner-Cremer, D. McCarroll, N.J. Loader, I. Robertson, R.
Jalkanen, G. Young, and A. Kirchhefer, 2011: Evidence of changing intrinsic
water-use efficiency under rising atmospheric CO
2
concentrations in Boreal
Fennoscandia from subfossil leaves and tree ring
13
C ratios. Global Change
Biology, 17(2), 1064-1072.
Galiano, L., J. Martínez-Vilalta, and F. Lloret, 2010: Drought-induced multifactor
decline of Scots pine in the Pyrenees and potential vegetation change by the
expansion of co-occurring oak species. Ecosystems, 13(7), 978-991.

338
Chapter 4 Terrestrial and Inland Water Systems
4
Gallant, D., B.G. Slough, D.G. Reid, and D. Berteaux, 2012: Arctic fox versus red fox in
the warming Arctic: four decades of den surveys in north Yukon. Polar Biology,
35(9), 1421-1431.
Galloway, J.N., A.R. Townsend, J.W. Erisman, M. Bekunda, Z.C. Cai, J.R. Freney, L.A.
Martinelli, S.P. Seitzinger, and M.A. Sutton, 2008: Transformation of the nitrogen
cycle: recent trends, questions, and potential solutions. Science, 320(5878),
889-892.
Ganey, J.L. and S.C. Vojta, 2011: Tree mortality in drought-stressed mixed-conifer and
ponderosa pine forests, Arizona, USA. Forest Ecology and Management, 261(1),
162-168.
Gao, J. and Y. Liu, 2011: Climate warming and land use change in Heilongjiang
Province, Northeast China. Applied Geography, 31(2), 476-482.
Gao, X.J. and F. Giorgi, 2008: Increased aridity in the Mediterranean region under
greenhouse gas forcing estimated from high resolution simulations with a
regional climate model. Global and Planetary Change, 62(3-4), 195-209.
Garreta, V., P.A. Miller, J. Guiot, C. Hely, S. Brewer, M.T. Sykes, and T. Litt, 2010: A
method for climate and vegetation reconstruction through the inversion of a
dynamic vegetation model. Climate Dynamics, 35(2-3), 371-389.
Garrity, S.R., C.D. Allen, S.P. Brumby, C. Gangodagamage, N.G. McDowell, and D.M.
Cai, 2013: Quantifying tree mortality in a mixed species woodland using
multitemporal high spatial resolution satellite imagery. Remote Sensing of
Environment, 129, 54-65.
Gaudnik, C., E. Corcket, B. Clement, C.E.L. Delmas, S. Gombert-Courvoisier, S. Muller,
C.J. Stevens, and D. Alard, 2011: Detecting the footprint of changing atmospheric
nitrogen deposition loads on acid grasslands in the context of climate change.
Global Change Biology, 17(11), 3351-3365.
Gauthier, G., J. Bety, M.-C. Cadieux, P. Legagneux, M. Doiron, C. Chevallier, S. Lai, A.
Tarroux, and D. Berteaux, 2013: Long-term monitoring at multiple trophic levels
suggests heterogeneity in responses to climate change in the Canadian Arctic
tundra. Philosophical Transactions of the Royal Society B, 368(1624), doi:
10.1098/rstb.2012.0482.
Gedalof, Z. and A.A. Berg, 2010: Tree ring evidence for limited direct CO
2
fertilization
of forests over the 20
th
century. Global Biogeochemical Cycles, 24(3), GB3027,
doi:10.1029/2009GB003699.
Gerten, D., S. Rost, W. von Bloh, and W. Lucht, 2008: Causes of change in 20
th
century
global river discharge. Geophysical Research Letters, 35(20), L20405,
doi:10.1029/2008GL035258.
Ghermandi, L., M.I.D. Curth, J. Franzese, and S. Gonzalez, 2010: Non-linear ecological
processes, fires, environmental heterogeneity and shrub invasion in northwestern
Patagonia. Ecological Modelling, 221(1), 113-121.
Giannakopoulos, C., P. Le Sager, M. Bindi, M. Moriondo, E. Kostopoulou, and C.M.
Goodess, 2009: Climatic changes and associated impacts in the Mediterranean
resulting from a 2 degrees C global warming. Global and Planetary Change,
68(3), 209-224.
Gibson, D.J. and L.C. Hulbert, 1987: Effects of fire, topography and year-to-year
climatic variation on species composition in tallgrass prairie. Vegetatio, 72(3),
175-185.
Gibson, L., T.M. Lee, L.P. Koh, B.W. Brook, T.A. Gardner, J. Barlow, C.A. Peres, C.J.A.
Bradshaw, W.F. Laurance, T.E. Lovejoy, and N.S. Sodhi, 2011: Primary forests are
irreplaceable for sustaining tropical biodiversity. Nature, 478(7369), 378- 381.
Gienapp, P., C. Teplitsky, J.S. Alho, J.A. Mills, and J. Merila, 2008: Climate change and
evolution: disentangling environmental and genetic responses. Molecular
Ecology, 17(1), 167-178.
Gienapp, P., M. Lof, T.E. Reed, J. McNamara, S. Verhulst, and M.E. Visser, 2013:
Predicting demographically sustainable rates of adaptation: can great tit
breeding time keep pace with climate change? Philosophical Transactions of
the Royal Society B, 368(1610), 20120289, doi:10.1098/rstb.2012.0289.
Giglio, L., J.T. Randerson, and G.R. van der Werf, 2013: Analysis of daily, monthly, and
annual burned area using the fourth-generation global fire emissions database
(GFED4). Journal of Geophysical Research: Biogeosciences, 118(1), 317-328.
Gilg, O., K.M. Kovacs, J. Aars, J. Fort, G. Gauthier, D. Gremillet, R.A. Ims, H. Meltofte,
J. Moreau, E. Post, N.M. Schmidt, G. Yannic, and L. Bollache, 2012: Climate
change and the ecology and evolution of Arctic vertebrates. In: The Year in Ecology
and Conservation Biology 2012 [Ostfeld, R.S. and W.H. Schlesinger (eds.)]. Vol. 102
of the Annals of the New York Academy of Sciences, Wiley-Blackwell Publishing
(for the New York Academy of Science), Boston, MA, USA, pp. 166-190.
Gill, A.M., J.Z. Wionarski, and A. York, 1999: Australians Biodiversity Responses to Fire:
Plants, Birds and Invertebrates. Commonwealth Department of the Environment
and Heritage, Canberra, Australia, 267 pp.
Gill, J.L., J.W. Williams, S.T. Jackson, K.B. Lininger, and G.S. Robinson, 2009: Pleistocene
megafaunal collapse, novel plant communities, and enhanced fire regimes in
North America. Science, 326(5956), 1100-1103.
Gillingham, P.K., B. Huntley, W.E. Kunin, and C.D. Thomas, 2012: The effect of spatial
resolution on projected responses to climate warming. Diversity and Distributions,
18(10), 990-1000.
Gilman, S.E., M.C. Urban, J. Tewksbury, G.W. Gilchrist, and R.D. Holt, 2010: A
framework for community interactions under climate change. Trends in Ecology
& Evolution, 25(6), 325-331.
Giorgi, F. and P. Lionello, 2008: Climate change projections for the Mediterranean
region. Global and Planetary Change, 63(2-3), 90-104.
Girardin, M.P. and M. Mudelsee, 2008: Past and future changes in Canadian boreal
wildfire activity. Ecological Applications, 18(2), 391-406.
Girardin, M.P., A.A. Ali, C. Carcaillet, M. Mudelsee, I. Drobyshev, C. Hely, and Y.
Bergeron, 2009: Heterogeneous response of circumboreal wildfire risk to climate
change since the early 1900s. Global Change Biology, 15(11), 2751-2769.
Girardin, M.P., P.Y. Bernier, and S. Gauthier, 2011: Increasing potential NEP of eastern
boreal North American forests constrained by decreasing wildfire activity.
Ecosphere, 2, 25, doi:10.1890/ES10-00159.1.
Girardin, M.P., X.J. Guo, P.Y. Bernier, F. Raulier, and S. Gauthier, 2012: Changes in
growth of pristine boreal North American forests from 1950 to 2005 driven by
landscape demographics and species traits. Biogeosciences, 9(7), 2523-2536.
Girardin, M.P., A.A. Ali, C. Carcaillet, S. Gauthier, C. Hely, H. Le Goff, A. Terrier, and Y.
Bergeron, 2013a: Fire in managed forests of eastern Canada: risks and options.
Forest Ecology and Management, 294, 238-249.
Girardin, M.P., A.A. Ali, C. Carcaillet, O. Blarquez, C. Hely, A. Terrier, A. Genries, and
Y. Bergeron, 2013b: Vegetation limits the impact of a warm climate on boreal
wildfires. New Phytologist, 199(4), 1001-1011.
Goetz, S.J., M.C. Mack, K.R. Gurney, J.T. Randerson, and R.A. Houghton, 2007:
Ecosystem responses to recent climate change and fire disturbance at northern
high latitudes: observations and model results contrasting northern Eurasia
and North America. Environmental Research Letters, 2(4), 045031, doi:10.1088/
1748-9326/2/4/045031.
Goetz, S.J., H.E. Epstein, U. Bhatt, G.J. Jia, J.O. Kaplan, H. Lischke, Q. Yu, A. Bunn, A.
Lloyd, D. Alcaraz, P.S.A. Beck, J. Comiso, M.K. Raynolds, and D.A. Walker, 2011:
Recent changes in Arctic vegetation: satellite observations and simulation
model predictions. In: Eurasian Arctic Land Cover and Land Use in a Changing
Climate [Gutman, G. and A. Reissell (eds.)]. Springer-Verlag, Amsterdam,
Netherlands, pp. 9-36.
Goldblum, D. and L.S. Rigg, 2010: The deciduous forest – boreal forest ecotone.
Geography Compass, 4(7), 701-717.
Golding, N. and R. Betts, 2008: Fire risk in Amazonia due to climate change in the
HadCM3 climate model: potential interactions with deforestation. Global
Biogeochemical Cycles, 22(4), GB4007, doi:10.1029/2007GB003166.
Gonzalez, P., 2001: Desertification and a shift of forest species in the West African
Sahel. Climate Research, 17(2), 217-228.
Gonzalez, P., R.P. Neilson, J.M. Lenihan, and R.J. Drapek, 2010: Global patterns in
the vulnerability of ecosystems to vegetation shifts due to climate change.
Global Ecology and Biogeography, 19(6), 755-768.
Gonzalez, P., C.J. Tucker, and H. Sy, 2012: Tree density and species decline in the
African Sahel attributable to climate. Journal of Arid Environments, 78(0), 55-64.
Good, P., C. Jones, J. Lowe, R. Betts, B. Booth, and C. Huntingford, 2011a: Quantifying
environmental drivers of future tropical forest extent. Journal of Climate, 24(5),
1337-1349.
Good, P., J. Caesar, D. Bernie, J.A. Lowe, P. van der Linden, S.N. Gosling, R. Warren,
N.W. Arnell, S. Smith, J. Bamber, T. Payne, S. Laxon, M. Srokosz, S. Sitch, N. Gedney,
G. Harris, H. Hewitt, L. Jackson, C.D. Jones, F. O’Connor, J. Ridley, M. Vellinga, P.
Halloran, and D. McNeall, 2011b: A review of recent developments in climate
change science. Part I: understanding of future change in the large-scale climate
system. Progress in Physical Geography, 35(3), 281-296.
Good, P., C. Jones, J. Lowe, R. Betts, and N. Gedney, 2013: Comparing tropical forest
projections from two generations of Hadley Centre Earth System Models,
HadGEM2-ES and HadCM3LC. Journal of Climate, 26(2), 495-511.
Gordo, O., 2007: Why are bird migration dates shifting? A review of weather and
climate effects on avian migratory phenology. Climate Research, 35(1-2), 37-58.
Gordo, O. and J.J. Sanz, 2005: Phenology and climate change: a long-term study in
a Mediterranean locality. Oecologia, 146(3), 484-495.
Gordo, O. and J.J. Sanz, 2010: Impact of climate change on plant phenology in
Mediterranean ecosystems. Global Change Biology, 16(3), 1082-1106.

339
Terrestrial and Inland Water Systems Chapter 4
4
Gottfried, M., H. Pauli, A. Futschik, M. Akhalkatsi, P. Barancok, J.L.B. Alonso, G. Coldea,
J. Dick, B. Erschbamer, M.R.F. Calzado, G. Kazakis, J. Krajci, P. Larsson, M.
Mallaun, O. Michelsen, D. Moiseev, P. Moiseev, U. Molau, A. Merzouki, L. Nagy,
G. Nakhutsrishvili, B. Pedersen, G. Pelino, M. Puscas, G. Rossi, A. Stanisci, J.P.
Theurillat, M. Tomaselli, L. Villar, P. Vittoz, I. Vogiatzakis, and G. Grabherr, 2012:
Continent-wide response of mountain vegetation to climate change. Nature
Climate Change, 2(2), 111-115.
Graiprab, P., K. Pongput, N. Tangtham, and P.W. Gassman, 2010: Hydrologic evaluation
and effect of climate change on the At Samat watershed, Northeastern Region,
Thailand. International Agricultural Engineering Journal, 19(2), 12-22.
Green, R.E., Y.C. Collingham, S.G. Willis, R.D. Gregory, K.W. Smith, and B. Huntley, 2008:
Performance of climate envelope models in retrodicting recent changes in bird
population size from observed climatic change. Biology Letters, 4(5), 599-602.
Griesbauer, H.P. and D.S. Green, 2012: Geographic and temporal patterns in white
spruce climate – growth relationships in Yukon, Canada. Forest Ecology and
Management, 267, 215-227.
Grime, J.P., J.D. Fridley, A.P. Askew, K. Thompson, J.G. Hodgson, and C.R. Bennet, 2008:
Long-term resistance to simulated climate change in an infertile grassland.
Proceedings of the National Academy of Sciences of the United States of
America, 105(29), 10028-10032.
Groisman, P.Y., R.W. Knight, and T.R. Karl, 2012: Changes in intense precipitation
over the central United States. Journal of Hydrometeorology, 13(1), 47-66.
Grosse, G., J. Harden, M. Turetsky, A.D. McGuire, P. Camill, C. Tarnocai, S. Frolking,
E.A.G. Schuur, T. Jorgenson, S. Marchenko, V. Romanovsky, K.P. Wickland, N.
French, M. Waldrop, L. Bourgeau-Chavez, and R.G. Striegl, 2011: Vulnerability
of high-latitude soil organic carbon in North America to disturbance. Journal
of Geophysical Research: Biogeosciences, 116(G4), G00K06, doi:10.1029/
2010JG001507.
Gruber, N. and J.N. Galloway, 2008: An Earth-system perspective of the global
nitrogen cycle. Nature, 451(7176), 293-296.
Guglielmin, M. and N. Cannone, 2012: A permafrost warming in a cooling
Antarctica? Climatic Change, 111(2), 177-195.
Gunderson, A.R. and M. Leal, 2012: Geographic variation in vulnerability to climate
warming in a tropical Caribbean lizard. Functional Ecology, 26(4), 783-793.
Gunderson, C.A., N.T. Edwards, A.V. Walker, K.H. O’Hara, C.M. Campion, and P.J. Hanson,
2012: Forest phenology and a warmer climate – growing season extension in
relation to climatic provenance. Global Change Biology, 18(6), 2008-2025.
Gunderson, L. and C.S. Holling (eds.), 2001: Panarchy: Understanding Transformations
in Systems of Humans and Nature. Island Press, Washington, DC, USA, 507 pp.
Gyllström, M., L.A. Hansson, E. Jeppesen, F. Garcia-Criado, E. Gross, K. Irvine, T.
Kairesalo, R. Kornijow, M.R. Miracle, M. Nykanen, T. Noges, S. Romo, D. Stephen,
E. Van Donk, and B. Moss, 2005: The role of climate in shaping zooplankton
communities of shallow lakes. Limnology and Oceanography, 50(6), 2008-2021.
Haberl, H., K.H. Erb, F. Krausmann, A. Bondeau, C. Lauk, C. Müller, C. Plutzar, and J.K.
Steinberger, 2011: Global bioenergy potentials from agricultural land in 2050:
Sensitivity to climate change, diets and yields. Biomass and Bioenergy, 35(12),
4753-4769.
Hague, M.J., M.R. Ferrari, J.R. Miller, D.A. Patterson, G.L. Russell, A.P. Farrell, and S.G.
Hinch, 2011: Modelling the future hydroclimatology of the lower Fraser River
and its impacts on the spawning migration survival of sockeye salmon. Global
Change Biology, 17(1), 87-98.
Haider, S., C. Kueffer, P.J. Edwards, and J.M. Alexander, 2012: Genetically based
differentiation in growth of multiple non-native plant species along a steep
environmental gradient. Oecologia, 170(1), 89-99.
Hall, J.S., M.S. Ashton, E.J. Garen, and S. Jose, 2011: The ecology and ecosystem services
of native trees: implications for reforestation and land restoration in Mesoamerica.
Forest Ecology and Management, 261(10), 1553-1557.
Halley, J.M., Y. Iwasa, and D. Vokou, 2013: Comment on “Extinction debt and windows
of conservation opportunity in the Brazilian Amazon”. Science, 339(6117), 271.
Hamilton, S.K., 2010: Biogeochemical implications of climate change for tropical
rivers and floodplains. Hydrobiologia, 657(1), 19-35.
Hampe, A., 2011: Plants on the move: the role of seed dispersal and initial population
establishment for climate-driven range expansions. Acta Oecologica:
International Journal of Ecology, 37(6), 666-673.
Hannah, L., 2012: Saving a Million Species: Extinction Risk from Climate Change.
Island Press, Washington, DC, USA, 419 pp.
Hannah, L., G. Midgley, S. Andelman, M. Araujo, G. Hughes, E. Martinez-Meyer, R.
Pearson, and P. Williams, 2007: Protected area needs in a changing climate.
Frontiers in Ecology and the Environment, 5(3), 131-138.
Hansen, M.M., I. Olivieri, D.M. Waller, E.E. Nielsen, and The GeM Working Group,
2012: Monitoring adaptive genetic responses to environmental change.
Molecular Ecology, 21(6), 1311-1329.
Hari, P. and L. Kulmata, 2008: Boreal Forest and Climate Change. Springer, New York,
NY, USA, 582 pp.
Harris, J.A., R.J. Hobbs, E. Higgs, and J. Aronson, 2006: Ecological restoration and
global climate change. Restoration Ecology, 14, 170-176.
Harrison, S.P. and M.F.S. Goni, 2010: Global patterns of vegetation response to
millennial-scale variability and rapid climate change during the last glacial
period. Quaternary Science Reviews, 29(21-22), 2957-2980.
Harsch, M.A., P.E. Hulme, M.S. McGlone, and R.P. Duncan, 2009: Are treelines
advancing? A global meta-analysis of treeline response to climate warming.
Ecology Letters, 12(10), 1040-1049.
Hastings, A., 2004: Transients: the key to long-term ecological understanding? Trends
in Ecology & Evolution, 19(1), 39-45.
Haxeltine, A. and I.C. Prentice, 1996: BIOME3: an equilibrium terrestrial biosphere
model based on ecophysiological constraints, resource availability, and
competition among plant functional types. Global Biogeochemical Cycles, 10,
693-709.
Hayes, F., M.L.M. Jones, G. Mills, and M. Ashmore, 2007: Meta-analysis of the relative
sensitivity of semi-natural vegetation species to ozone. Environmental Pollution,
146(3), 754-762.
Hayes, K.R. and S.C. Barry, 2008: Are there any consistent predictors of invasion
success? Biological Invasions, 10(4), 483-506.
Haywood, A.M. and P.J. Valdes, 2006: Vegetation cover in a warmer world simulated
using a dynamic global vegetation model for the Mid-Pliocene. Palaeogeography
Palaeoclimatology Palaeoecology, 237(2-4), 412-427.
Haywood, A.M., A. Ridgwell, D.J. Lunt, D.J. Hill, M.J. Pound, H.J. Dowsett, A.M. Dolan,
J.E. Francis, and M. Williams, 2011: Are there pre-Quaternary geological analogues
for a future greenhouse warming? Philosophical Transactions of the Royal
Society A, 369(1938), 933-956.
Heckenberger, M.J., J.C. Russell, J.R. Toney, and M.J. Schmidt, 2007: The legacy of
cultural landscapes in the Brazilian Amazon: implications for biodiversity.
Philosophical Transactions of the Royal Society B, 362(1478), 197-208.
Hegarty, M.J., 2012: Invasion of the hybrids. Molecular Ecology, 21(19), 4669-4671.
Hegglin, M.I. and T.G. Shepherd, 2009: Large climate-induced changes in ultraviolet
index and stratosphere-to-troposphere ozone flux. Nature Geoscience, 10(10),
687- 691.
Hegland, S.J., A. Nielsen, A. Lazaro, A.L. Bjerknes, and Totland, 2009: How does climate
warming affect plant-pollinator interactions? Ecology Letters, 12(2), 184-195.
Hejcman, M., P. Hejcmanova, V. Pavlu, and J. Benes, 2013: Origin and history of grasslands
in Central Europe – a review. Grass and Forage Science, 68(3), 345-363.
Hellden, U. and C. Tottrup, 2008: Regional desertification: a global synthesis. Global
and Planetary Change, 64(3-4), 169-176.
Heller, N.E. and E.S. Zavaleta, 2009: Biodiversity management in the face of climate
change: a review of 22 years of recommendations. Biological Conservation,
142(1), 14-32.
Hellmann, J.J., J.E. Byers, B.G. Bierwagen, and J.S. Dukes, 2008: Five potential
consequences of climate change for invasive species. Conservation Biology,
22(3), 534-543.
Hemery, G.E., 2008: Forest management and silvicultural responses to projected climate
change impacts on European broadleaved trees and forests. International
Forestry Review, 10(4), 591-607.
Hendry, A.P. and A. Gonzalez, 2008: Whither adaptation? Biology & Philosophy,
23(5), 673-699.
Hermoso, V. and M. Clavero, 2011: Threatening processes and conservation
management of endemic freshwater fish in the Mediterranean basin: a review.
Marine and Freshwater Research, 62(3), 244-254.
Hewitt, N., N. Klenk, A.L. Smith, D.R. Bazely, N. Yan, S. Wood, J.I. MacLellan, C. Lipsig-
Mumme, and I. Henriques, 2011: Taking stock of the assisted migration debate.
Biological Conservation, 144(11), 2560-2572.
Hickler, T., B. Smith, I.C. Prentice, K. Mjöfors, P. Miller, A. Arneth, and M.T. Sykes, 2008:
CO
2
fertilization in temperate FACE experiments not representative of boreal
and tropical forests. Global Change Biology, 14, 1531-1542.
Hickler, T., K. Vohland, J. Feehan, P.A. Miller, B. Smith, L. Costa, T. Giesecke, S. Fronzek,
T.R. Carter, W. Cramer, I. Kühn, and M.T. Sykes, 2012: Projecting the future
distribution of European potential natural vegetation zones with a generalized,
tree species-based dynamic vegetation model. Global Ecology and Biogeography,
21(1), 50-63.

340
Chapter 4 Terrestrial and Inland Water Systems
4
Hickling, R., D.B. Roy, J.K. Hill, R. Fox, and C.D. Thomas, 2006: The distributions of a
wide range of taxonomic groups are expanding polewards. Global Change
Biology, 12(3), 450-455.
Higgins, S.I. and S. Scheiter, 2012: Atmospheric CO
2
forces abrupt vegetation shifts
locally, but not globally. Nature, 488(7410), 209-212.
Higgins, S.I., J.S. Clark, R. Nathan, T. Hovestadt, F. Schurr, J.M.V. Fragoso, M.R. Aguiar,
E. Ribbens, and S. Lavorel, 2003: Forecasting plant migration rates: managing
uncertainty for risk assessment. Journal of Ecology, 91(3), 341-347.
Higgins, S.I., R.B. O’Hara, and C. Romermann, 2012: A niche for biology in species
distribution models. Journal of Biogeography, 39(12), 2091-2095.
Higuera, P.E., M.L. Chipman, J.L. Barnes, M.A. Urban, and F.S. Hu, 2011: Variability of
tundra fire regimes in Arctic Alaska: millennial-scale patterns and ecological
implications. Ecological Applications, 21(8), 3211-3226.
Hijioka, Y., Y. Matsuoka, H. Nishimoto, and M. Kainuma, (2008): Global GHG emission
scenrios under GHG concentration stabilization targets. Journal of Global
Environmental Engineering, 13, 97-108.
Hill, P.W., J. Farrar, P. Roberts, M. Farrell, H. Grant, K.K. Newsham, D.W. Hopkins, R.D.
Bardgett, and D.L. Jones, 2011: Vascular plant success in a warming Antarctic
may be due to efficient nitrogen acquisition. Nature Climate Change, 1(1), 50-
53.
Hinzman, L.D., N.D. Bettez, W.R. Bolton, F.S. Chapin III, M.B. Dyurgerov, C.L. Fastie, B.
Griffith, R.D. Hollister, A. Hope, H.P. Huntington, A.M. Jensen, G.J. Jia, T. Jorgenson,
D.L. Kane, D.R. Klein, G. Kofinas, A.H. Lynch, A.H. Lloyd, A.D. McGuire, F.E. Nelson,
W.C. Oechel, T.E. Osterkamp, C.H. Racine, V.E. Romanovsky, R.S. Stone, D.A.
Stow, M. Sturm, C.E. Tweedie, G.L. Vourlitis, M.D. Walker, D.A. Walker, P.J. Webber,
J.M. Welker, K. Winker, and K. Yoshikawa, 2005: Evidence and implications of
recent climate change in northern Alaska and other arctic regions. Climatic
Change, 72(3), 251-298.
Hockey, P.A.R., C. Sirami, A.R. Ridley, G.F. Midgley, and H.A. Babiker, 2011:
Interrogating recent range changes in South African birds: confounding signals
from land use and climate change present a challenge for attribution. Diversity
and Distributions, 17(2), 254-261.
Hodgson, J.A., C.D. Thomas, C. Dytham, J.M.J. Travis, and S.J. Cornell, 2012: The speed
of range shifts in fragmented landscapes. PLoS One, 7(10), e47141, doi:10.1371/
journal.pone.0047141.
Hoegh-Guldberg, O., L. Hughes, S. McIntyre, D.B. Lindenmayer, C. Parmesan, H.P.
Possingham, and C.D. Thomas, 2008: Assisted colonization and rapid climate
change. Science, 321(5887), 345-346.
Hof, C., M.B. Araujo, W. Jetz, and C. Rahbek, 2011a: Additive threats from pathogens,
climate and land-use change for global amphibian diversity. Nature,
480(7378), 516-519.
Hof, C., I. Levinsky, M.B. Araujo, and C. Rahbek, 2011b: Rethinking species’ ability to
cope with rapid climate change. Global Change Biology, 17(9), 2987-2990.
Hoffmann, A.A. and C.M. Sgro, 2011: Climate change and evolutionary adaptation.
Nature, 470(7335), 479-485.
Hoffmann, M., C. Hilton-Taylor, A. Angulo, M. Bohm, T.M. Brooks, S.H.M. Butchart,
K.E. Carpenter, J. Chanson, B. Collen, N.A. Cox, W.R.T. Darwall, N.K. Dulvy, L.R.
Harrison, V. Katariya, C.M. Pollock, S. Quader, N.I. Richman, A.S.L. Rodrigues,
M.F. Tognelli, J.C. Vie, J.M. Aguiar, D.J. Allen, G.R. Allen, G. Amori, N.B. Ananjeva,
F. Andreone, P. Andrew, A.L.A. Ortiz, J.E.M. Baillie, R. Baldi, B.D. Bell, S.D. Biju,
J.P. Bird, P. Black-Decima, J.J. Blanc, F. Bolanos, W. Bolivar, I.J. Burfield, J.A. Burton,
D.R. Capper, F. Castro, G. Catullo, R.D. Cavanagh, A. Channing, N.L. Chao, A.M.
Chenery, F. Chiozza, V. Clausnitzer, N.J. Collar, L.C. Collett, B.B. Collette, C.F.C.
Fernandez, M.T. Craig, M.J. Crosby, N. Cumberlidge, A. Cuttelod, A.E. Derocher,
A.C. Diesmos, J.S. Donaldson, J.W. Duckworth, G. Dutson, S.K. Dutta, R.H. Emslie,
A. Farjon, S. Fowler, J. Freyhof, D.L. Garshelis, J. Gerlach, D.J. Gower, T.D. Grant,
G.A. Hammerson, R.B. Harris, L.R. Heaney, S.B. Hedges, J.M. Hero, B. Hughes,
S.A. Hussain, J. Icochea, R.F. Inger, N. Ishii, D.T. Iskandar, R.K.B. Jenkins, Y. Kaneko,
M. Kottelat, K.M. Kovacs, S.L. Kuzmin, E. La Marca, J.F. Lamoreux, M.W.N. Lau,
E.O. Lavilla, K. Leus, R.L. Lewison, G. Lichtenstein, S.R. Livingstone, V. Lukoschek,
D.P. Mallon, P.J.K. McGowan, A. McIvor, P.D. Moehlman, S. Molur, A.M. Alonso,
J.A. Musick, K. Nowell, R.A. Nussbaum, W. Olech, N.L. Orlov, T.J. Papenfuss, G.
Parra-Olea, W.F. Perrin, B.A. Polidoro, M. Pourkazemi, P.A. Racey, J.S. Ragle, M.
Ram, G. Rathbun, R.P. Reynolds, A.G.J. Rhodin, S.J. Richards, L.O. Rodriguez, S.R.
Ron, C. Rondinini, A.B. Rylands, Y.S. de Mitcheson, J.C. Sanciangco, K.L. Sanders,
G. Santos-Barrera, J. Schipper, C. Self-Sullivan, Y.C. Shi, A. Shoemaker, F.T. Short,
C. Sillero-Zubiri, D.L. Silvano, K.G. Smith, A.T. Smith, J. Snoeks, A.J. Stattersfield,
A.J. Symes, A.B. Taber, B.K. Talukdar, H.J. Temple, R. Timmins, J.A. Tobias, K.
Tsytsulina, D. Tweddle, C. Ubeda, S.V. Valenti, P.P. van Dijk, L.M. Veiga, A. Veloso,
D.C. Wege, M. Wilkinson, E.A. Williamson, F. Xie, B.E. Young, H.R. Akcakaya, L.
Bennun, T.M. Blackburn, L. Boitani, H.T. Dublin, G.A.B. da Fonseca, C. Gascon,
T.E. Lacher, G.M. Mace, S.A. Mainka, J.A. McNeely, R.A. Mittermeier, G.M. Reid,
J.P. Rodriguez, A.A. Rosenberg, M.J. Samways, J. Smart, B.A. Stein, and S.N. Stuart,
2010: The impact of conservation on the status of the World’s vertebrates.
Science, 330(6010), 1503-1509.
Hofmann, G.E. and A.E. Todgham, 2010: Living in the now: physiological mechanisms
to tolerate a rapidly changing environment. Annual Review of Physiology, 72,
127-145.
Hofmockel, K.S., D.R. Zak, K.K. Moran, and J.D. Jastrow, 2011: Changes in forest soil
organic matter pools after a decade of elevated CO
2
and O
3
. Soil Biology and
Biochemistry, 43(7), 1518-1527.
Hogg, E.H., J.P. Brandt, and M. Michaelian, 2008: Impact of a regional drought on
the productivity, dieback and biomass of western Canadian aspen forests.
Canadian Journal of Forest Research / Revue Canadienne De Recherche
Forestiere, 38, 1373-1384.
Hole, D.G., S.G. Willis, D.J. Pain, L.D. Fishpool, S.H.M. Butchart, Y.C. Collingham, C.
Rahbek, and B. Huntley, 2009: Projected impacts of climate change on a
continent-wide protected area network. Ecology Letters, 12(5), 420-431.
Hole, D.G., B. Huntley, J. Arinaitwe, S.H.M. Butchart, Y.C. Collingham, L.D.C. Fishpool,
D.J. Pain, and S.G. Willis, 2011: Toward a management framework for networks
of protected areas in the face of climate change. Conservation Biology, 25(2),
305-315.
Holmgren, M., P. Stapp, C.R. Dickman, C. Gracia, S. Graham, J.R. Gutiérrez, C. Hice,
F. Jaksic, D.A. Kelt, M. Letnic, M. Lima, B.C. López, P.L. Meserve, W.B. Milstead,
G.A. Polis, M.A. Previtali, M. Richter, S. Sabaté, and F.A. Squeo, 2006: Extreme
climatic events shape arid and semiarid ecosystems. Frontiers in Ecology and
the Environment, 4(2), 87-95.
Hongve, D., G. Riise, and J.F. Kristiansen, 2004: Increased colour and organic acid
concentrations in Norwegian forest lakes and drinking water – a result of
increased precipitation? Aquatic Sciences, 66(2), 231-238.
Hooijer, A., S. Page, J.G. Canadell, M. Silvius, J. Kwadijk, H. Wosten, and J. Jauhiainen,
2010: Current and future CO
2
emissions from drained peatlands in Southeast
Asia. Biogeosciences, 7(5), 1505-1514.
Hoover, S.E.R., J.J. Ladley, A.A. Shchepetkina, M. Tisch, S.P. Gieseg, and J.M. Tylianakis,
2012: Warming, CO
2
, and nitrogen deposition interactively affect a plant-
pollinator mutualism. Ecology Letters, 15(3), 227-234.
Horowitz, L.W., 2006: Past present and future concentrations of tropospheric ozone
and aerosols: morthodology, ozone evaluation, and sensitivity to aerosol wet
removal. Journal of Geophysical Research: Atmospheres, 111(D22), D22211,
doi:10.1029/2005JD006937 .
Hosonuma, N., M. Herold, V. De Sy, R.S. De Fries, M. Brockhaus, L. Verchot, A.
Angelsen, and E. Romijn, 2012: An assessment of deforestation and forest
degradation drivers in developing countries. Environmental Research Letters,
7(4), 044009, doi:10.1088/1748-9326/7/4/044009.
Høye, T.T., E. Post, H. Meltofte, N.M. Schmidt, and M.C. Forchhammer, 2007: Rapid
advancement of spring in the High Arctic. Current Biology, 17(12), R449-
R451.
Hoyle, C.R., M. Boy, N.M. Donahue, J.L. Fry, M. Glasius, A. Guenther, A.G. Hallar, K.H.
Hartz, M.D. Petters, T. Petaja, T. Rosenoern, and A.P. Sullivan, 2011: A review of the
anthropogenic influence on biogenic secondary organic aerosol. Atmospheric
Chemistry and Physics, 11(1), 321-343.
Huang, D., R.A. Haack, and R. Zhang, 2011: Does global warming increase establishment
rates of invasive alien species? A Centurial time series analysis. PLoS One, 6(9),
doi:10.1371/journal.pone.0024733.
Huang, J.G., Y. Bergeron, B. Denneler, F. Berninger, and J. Tardif, 2007: Response of
forest trees to increased atmospheric CO
2
. Critical Reviews in Plant Sciences,
26(5-6), 265-283.
Huey, R.B., M.R. Kearney, A. Krockenberger, J.A.M. Holtum, M. Jess, and S.E. Williams,
2012: Predicting organismal vulnerability to climate warming: roles of behaviour,
physiology and adaptation. Philosophical Transactions of the Royal Society B,
367(1596), 1665-1679.
Hughes, R.F., S.R. Archer, G.P. Asner, C.A. Wessman, C.H.A.D. McMurtry, J.I.M. Nelson,
and R.J. Ansley, 2006: Changes in aboveground primary production and carbon
and nitrogen pools accompanying woody plant encroachment in a temperate
savanna. Global Change Biology, 12(9), 1733-1747.
Hughes, T.P., C. Linares, V. Dakos, I.A. van de Leemput, and E.H. van Nes, 2013: Living
dangerously on borrowed time during slow, unrecognized regime shifts. Trends
in Ecology & Evolution, 28(3), 149-155.

341
Terrestrial and Inland Water Systems Chapter 4
4
Hülber, K., M. Winkler, and G. Grabherr, 2010: Intraseasonal climate and habitat-
specific variability controls the flowering phenology of high alpine plant species.
Functional Ecology, 24(2), 245-252.
Hunt, A. and P. Watkiss, 2011: Climate change impacts and adaptation in cities: a
review of the literature. Climatic Change, 104(1), 13-49.
Hunter, C.M., H. Caswell, M.C. Runge, E.V. Regehr, S.C. Amstrup, and I. Stirling, 2010:
Climate change threatens polar bear populations: a stochastic demographic
analysis. Ecology, 91(10), 2883-2897.
Huntingford, C., P.M. Cox, L.M. Mercado, S. Sitch, N. Bellouin, O. Boucher, and N.
Gedney, 2011: Highly contrasting effects of different climate forcing agents on
terrestrial ecosystem services. Philosophical Transactions of the Royal Society
A, 369(1943), 2026-2037.
Huntingford, C., P. Zelazowski, D. Galbraith, L.M. Mercado, S. Sitch, R. Fisher, M.
Lomas, A. P.Walker, C.D. Jones, B.B.B. Booth, Y. Malhi, D. Hemming, G. Kay, P.
Good, S.L. Lewis, O.L. Phillips, O.K. Atkin, J. Lloyd, E. Gloor, J. Zaragoza-Castells,
P. Meir, R. Betts, P.P. Harris, C. Nobre, J. Marengo, and P.M. Cox, 2013: Simulated
resilience of tropical rainforests to CO
2
-induced climate change. Nature
Geoscience, 6, 268-273.
Huntington, T.G., 2008: CO
2
-induced suppression of transpiration cannot explain
increasing runoff. Hydrological Processes, 22(2), 311-314.
Hurtt, G.C., L.P. Chini, S. Frolking, R.A. Betts, J. Feddema, G. Fischer, J.P. Fisk, K. Hibbard,
R.A. Houghton, A. Janetos, C.D. Jones, G. Kindermann, T. Kinoshita, K.K. Goldewijk,
K. Riahi, E. Shevliakova, S. Smith, E. Stehfest, A. Thomson, P. Thornton, D.P.v.
Vuuren, and Y.P. Wang, 2011: Harmonization of land-use scenarios for the
period 1500–2100: 600 years of global gridded annual land-use transitions,
wood harvest, and resulting secondary lands. Climatic Change, 109, 117-161.
Husby, A., M.E. Visser, and L.E.B. Kruuk, 2011: Speeding up microevolution: the effects
of increasing temperature on selection and genetic variance in a wild bird
population. PLoS Biology, 9(2), e1000585, doi:10.1371/journal.pbio.1000585.
Ihlow, F., J. Dambach, J.O. Engler, M. Flecks, T. Hartmann, S. Nekum, H. Rajaei, and D.
Rodder, 2012: On the brink of extinction? How climate change may affect
global chelonian species richness and distribution. Global Change Biology,
18(5), 1520-1530.
Innes, J.L., 1992: Observations on the condition of beech (Fagus sylvatica L.) in Britain
in 1990. Forestry, 65(1), 35-60.
Inouye, D.W., 2008: Effects of climate change on phenology, frost damage, and floral
abundance of montane wildflowers. Ecology, 89(2), 353-362.
INPE, 2013: Projeto Desmatamento (PRODES): Monitoramento da Floresta Amazonica
por Satelite. Cited 2013, www.obt.inpe.br/prodes/.
IPCC, 2012: Summary for policymakers. In: Managing the Risks of Extreme Events
and Disasters to Advance Climate Change Adaptation. A Special Report of
Working Groups I and II of the Intergovernmental Panel on Climate Change
[Field, C.B., V. Barros, T.F. Stocker, D. Qin, D.J. Dokken, K.L. Ebi, M.D. Mastrandrea,
K.J. Mach, G.-K. Plattner, S.K. Allen, M. Tignor, and P.M. Midgley (eds.)].
Cambridge University Press, Cambridge, UK and New York, NY, USA, pp. 3-21.
Iverson, L.R., M.W. Schwartz, and A.M. Prasad, 2004: How fast and far might tree
species migrate in the eastern United States due to climate change? Global
Ecology and Biogeography, 13(3), 209-219.
Iverson, L.R., A. Prasad, and S. Matthews, 2008: Modeling potential climate change
impacts on the trees of the northeastern United States. Mitigation and
Adaptation Strategies for Global Change, 13(5-6), 487-516.
Iverson, L.R., A.M. Prasad, S.N. Matthews, and M.P. Peters, 2011: Lessons learned
while integrating habitat, dispersal, disturbance, and life-history traits into
species habitat models under climate change. Ecosystems, 14(6), 1005-1020.
Jackson, R.B., E.G. Jobbagy, R. Avissar, S.B. Roy, D.J. Barrett, C.W. Cook, K.A. Farley,
D.C. le Maitre, B.A. McCarl, and B.C. Murray, 2005: Trading water for carbon
with biological sequestration. Science, 310(5756), 1944-1947.
Jackson, S.T. and R.J. Hobbs, 2009: Ecological restoration in the light of ecological
history. Science, 325(5940), 567-569.
Jackson, S.T. and J.T. Overpeck, 2000: Responses of plant populations and communities
to environmental changes of the Late Quaternary. Paleobiology, 26(4), 194-
220.
Jackson, S.T., S.T. Gray, and B. Shuman, 2009: Paleoecology and resource management
in a dynamic landscape: case studies from the Rocky Mountain headwaters.
In: Conservation Paleobiology: Using the Past to Manage for the Future [Dietl,
G.P. and K.W. Flessa (eds.)]. The Paleontological Society Papers, Vol. 15, Boulder,
CO, USA, pp. 61-80.
Jacobsen, D., A.M. Milner, L.E. Brown, and O. Dangles, 2012: Biodiversity under threat
in glacier-fed river systems. Nature Climate Change, 2(5), 361-364.
Jamieson, M.A., A.M. Trowbridge, K.F. Raffa, and R.L. Lindroth, 2012: Consequences
of climate warming and altered precipitation patterns for plant-insect and
multitrophic interactions. Plant Physiology, 160(4), 1719-1727.
Jansen, E., J. Overpeck, K.R. Briffa, J.-C. Duplessy, F. Joos, V. Masson-Delmotte, D.
Olago, B. Otto-Bliesner, W.R. Peltier, S. Rahmstorf, R. Ramesh, D. Raynaud, D.
Rind, O. Solomina, R. Villalba, and D. Zhang, 2007: Paleoclimate. In: Climate
Change 2007: The Physical Science Basis. Contribution of Working Group I to
the Fourth Assessment Report of the Intergovernmental Panel on Climate
Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M.
Tignor, and H.L. Miller (eds.)]. Cambridge University Press, Cambridge, UK and
New York, NY, USA, pp. 433-497.
Jaramillo, C., D. Ochoa, L. Contreras, M. Pagani, H. Carvajal-Ortiz, L.M. Pratt, S. Krishnan,
A. Cardona, M. Romero, L. Quiroz, G. Rodriguez, M.J. Rueda, F. de la Parra, S. Moron,
W. Green, G. Bayona, C. Montes, O. Quintero, R. Ramirez, G. Mora, S. Schouten,
H. Bermudez, R. Navarrete, F. Parra, M. Alvaran, J. Osorno, J.L. Crowley, V. Valencia,
and J. Vervoort, 2010: Effects of rapid global warming at the Paleocene-Eocene
boundary on neotropical vegetation. Science, 330(6006), 957-961.
Jarvis, P.G., 1976: The interpretation of the variations in leaf water potential and
stomatal conductance found in canopies in the field. Philosophical Transactions
of the Royal Society B, 273, 593-610.
Jenerette, G.D., S.L. Harlan, W.L. Stefanov, and C.A. Martin, 2011: Ecosystem services
and urban heat riskscape moderation: water, green spaces, and social inequality
in Phoenix, AZ, USA. Ecological Applications, 21(7), 2637-2651.
Jenkins, K.M. and A.J. Boulton, 2007: Detecting impacts and setting restoration
targets in arid-zone rivers: aquatic micro-invertebrate responses to reduced
floodplain inundation. Journal of Applied Ecology, 44(4), 823-832.
Jensen, K.D., C. Beier, A. Michelsen, and B.A. Emmett, 2003: Effects of experimental
drought on microbial processes in two temperate heathlands at contrasting
water conditions. Applied Soil Ecology, 24(2), 165-176.
Jeong, S.J., C.H. Ho, H.J. Gim, and M.E. Brown, 2011: Phenology shifts at start vs. end
of growing season in temperate vegetation over the Northern Hemisphere for
the period 1982-2008. Global Change Biology, 17(7), 2385-2399.
Jia, B., Y. Ma, and K. Qiu, 2009: Dynamics of the vegetation coverage in recent 15
years in Yijinhuoluo County, Inner Mongolia, China. Arid Land Geography,
32(4), 481-487.
Jia, G.J., H.E. Epstein, and D.A. Walker, 2009: Vegetation greening in the Canadian
Arctic related to decadal warming. Journal of Environmental Monitoring,
11(12), 2231-2238.
Jin, J., S. Lu, S. Li, and N.L. Miller, 2010: Impact of land use change on the local climate
over the Tibetan Plateau. Advances in Meteorology, 2010, 837480, doi:10.1155/
2010/837480.
Jin, Y., J.T. Randerson, S.J. Goetz, P.S.A. Beck, M.M. Loranty, and M.L. Goulden, 2012:
The influence of burn severity on postfire vegetation recovery and albedo change
during early succession in North American boreal forests. Journal of Geophysical
Research: Biogeosciences, 117(G1), G01036, doi:10.1029/2011JG001886.
Johanson, C.M. and Q. Fu, 2009: Hadley cell widening: model simulations versus
observations. Journal of Climate, 22(10), 2713-2725.
Jöhnk, K.D., J. Huisman, J. Sharples, B. Sommeijer, P.M. Visser, and J.M. Stroom, 2008:
Summer heatwaves promote blooms of harmful cyanobacteria. Global Change
Biology, 14(3), 495-512.
Johnson, W.C., B.V. Millett, T. Gilmanov, R.A. Voldseth, G.R. Guntenspergen, and D.E.
Naugle, 2005: Vulnerability of northern prairie wetlands to climate change.
Bioscience, 55(10), 863-872.
Johnstone, J.F., T.N. Hollingsworth, F.S. Chapin, and M.C. Mack, 2010: Changes in
fire regime break the legacy lock on successional trajectories in Alaskan boreal
forest. Global Change Biology, 16(4), 1281-1295.
Jones, C., J. Lowe, S. Liddicoat, and R. Betts, 2009: Committed terrestrial ecosystem
changes due to climate change. Nature Geoscience, 2(7), 484-487.
Jones, C.D., J.K. Hughes, N. Bellouin, S.C. Hardiman, G.S. Jones, J. Knight, S. Liddicoat,
F.M. O’Connor, R.J. Andres, C. Bell, K.O. Boo, A. Bozzo, N. Butchart, P. Cadule,
K.D. Corbin, M. Doutriaux-Boucher, P. Friedlingstein, J. Gornall, L. Gray, P.R.
Halloran, G. Hurtt, W.J. Ingram, J.F. Lamarque, R.M. Law, M. Meinshausen, S.
Osprey, E.J. Palin, L.P. Chini, T. Raddatz, M.G. Sanderson, A.A. Sellar, A. Schurer,
P. Valdes, N. Wood, S. Woodward, M. Yoshioka, and M. Zerroukat, 2011: The
HadGEM2-ES implementation of CMIP5 centennial simulations. Geoscientific
Model Development, 4(3), 543-570.
Jones, M.C., S.R. Dye, J.K. Pinnegar, R. Warren, and W.W.L. Cheung, 2012: Modelling
commercial fish distributions: prediction and assessment using different
approaches. Ecological Modelling, 225, 133-145.

342
Chapter 4 Terrestrial and Inland Water Systems
4
Jongman, R.H.G., I.M. Bouwma, A. Griffioen, L. Jones-Walters, and A.M. Van Doorn,
2011: The Pan European Ecological Network: PEEN. Landscape Ecology, 26(3),
311-326.
Jonsson, B. and N. Jonsson, 2009: A review of the likely effects of climate change on
anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with
particular reference to water temperature and flow. Journal of Fish Biology,
75(10), 2381-2447.
Jorgenson, M.T., V. Romanovsky, J. Harden, Y. Shur, J. O’Donnell, E.A.G. Schuur, M.
Kanevskiy, and S. Marchenko, 2010: Resilience and vulnerability of permafrost
to climate change. Canadian Journal of Forest Research / Revue Canadienne
De Recherche Forestiere, 40(7), 1219-1236.
Joubert, D.F., A. Rothauge, and G.N. Smit, 2008: A conceptual model of vegetation
dynamics in the semiarid Highland savanna of Namibia, with particular reference
to bush thickening by Acacia mellifera. Journal of Arid Environments, 72(12),
2201-2210.
Jung, M., M. Reichstein, P. Ciais, S.I. Seneviratne, J. Sheffield, M.L. Goulden, G. Bonan,
A. Cescatti, J.Q. Chen, R. de Jeu, A.J. Dolman, W. Eugster, D. Gerten, D. Gianelle,
N. Gobron, J. Heinke, J. Kimball, B.E. Law, L. Montagnani, Q.Z. Mu, B. Mueller, K.
Oleson, D. Papale, A.D. Richardson, O. Roupsard, S. Running, E. Tomelleri, N.
Viovy, U. Weber, C. Williams, E. Wood, S. Zaehle, and K. Zhang, 2010: Recent
decline in the global land evapotranspiration trend due to limited moisture
supply. Nature, 467(7318), 951-954.
Kaiser, K.E., B.L. McGlynn, and R.E. Emanuel, 2012: Ecohydrology of an outbreak:
mountain pine beetle impacts trees in drier landscape positions first.
Ecohydrology, 6(3), 444-454.
Kane, J.M., K.A. Meinhardt, T. Chang, B.L. Cardall, R. Michalet, and T.G. Whitham,
2011: Drought-induced mortality of a foundation species (Juniperus
monosperma) promotes positive afterlife effects in understory vegetation. Plant
Ecology, 212(5), 733-741.
Kanniah, K.D., J. Beringer, P. North, and L. Hutley, 2012: Control of atmospheric
particles on diffuse radiation and terrestrial plant productivity. Progress in
Physical Geography, 36(2), 209-237.
Kappes, H. and P. Haase, 2012: Slow, but steady: dispersal of freshwater molluscs.
Aquatic Sciences, 74(1), 1-14.
Karell, P., K. Ahola, T. Karstinen, J. Valkama, and J.E. Brommer, 2011: Climate change
drives microevolution in a wild bird. Nature Communications, 2, 208,
doi:10.1038/ncomms1213.
Karlsson, J., P. Bystrom, J. Ask, P. Ask, L. Persson, and M. Jansson, 2009: Light limitation
of nutrient-poor lake ecosystems. Nature, 460(7254), 506-509.
Karnosky, D.F., K.S. Pregitzer, D.R. Zak, M.E. Kubiske, G.R. Hendrey, D. Weinstein, M.
Nosal, and K.E. Percy, 2005: Scaling ozone responses of forest trees to the
ecosystem level in a changing climate. Plant Cell and Environment, 28(8), 965-981.
Kasischke, E.S., D.L. Verbyla, T.S. Rupp, A.D. McGuire, K.A. Murphy, R. Jandt, J.L. Barnes,
E.E. Hoy, P.A. Duffy, M. Calef, and M.R. Turetsky, 2010: Alaska’s changing fire
regime – implications for the vulnerability of its boreal forests. Canadian
Journal of Forest Research / Revue Canadienne De Recherche Forestiere, 40(7),
1313-1324.
Kasson, M.T. and W.H. Livingston, 2012: Relationships among beech bark disease,
climate, radial growth response and mortality of American beech in northern
Maine, USA. Forest Pathology, 42(3), 199-212.
Kaufman, D.S., D.P. Schneider, N.P. McKay, C.M. Ammann, R.S. Bradley, K.R. Briffa,
G.H. Miller, B.L. Otto-Bliesner, J.T. Overpeck, B.M. Vinther, and Arctic Lakes 2k
Project, 2009: Recent warming reverses long-term arctic cooling. Science,
325(5945), 1236-1239.
Kaushal, S.S., G.E. Likens, N.A. Jaworski, M.L. Pace, A.M. Sides, D. Seekell, K.T. Belt,
D.H. Secor, and R.L. Wingate, 2010: Rising stream and river temperatures in the
United States. Frontiers in Ecology and the Environment, 8(9), 461-466.
Kearney, M. and W. Porter, 2009: Mechanistic niche modelling: combining physiological
and spatial data to predict species’ ranges. Ecology Letters, 12(4), 334-350.
Kearney, M., W.P. Porter, C. Williams, S. Ritchie, and A.A. Hoffmann, 2009: Integrating
biophysical models and evolutionary theory to predict climatic impacts on
species’ ranges: the dengue mosquito Aedes aegypti in Australia. Functional
Ecology, 23(3), 528-538.
Kearney, M.R., B.A. Wintle, and W.P. Porter, 2010: Correlative and mechanistic models
of species distribution provide congruent forecasts under climate change.
Conservation Letters, 3(3), 203-213.
Keenan, T., J. Maria Serra, F. Lloret, M. Ninyerola, and S. Sabate, 2011: Predicting the
future of forests in the Mediterranean under climate change, with niche- and
process-based models: CO
2
matters! Global Change Biology, 17(1), 565-579.
Keith, D.A., H.R. Akcakaya, W. Thuiller, G.F. Midgley, R.G. Pearson, S.J. Phillips, H.M.
Regan, M.B. Araujo, and T.G. Rebelo, 2008: Predicting extinction risks under climate
change: coupling stochastic population models with dynamic bioclimatic habitat
models. Biology Letters, 4(5), 560-563.
Keith, H., E. van Gorsel, K.L. Jacobsen, and H.A. Cleugh, 2012: Dynamics of carbon
exchange in a Eucalyptus forest in response to interacting disturbance factors.
Agricultural and Forest Meteorolgy, 153, 67-81.
Ketola, T., V. Kellermann, T.N. Kristensen, and V. Loeschcke, 2012: Constant, cycling,
hot and cold thermal environments: strong effects on mean viability but not
on genetic estimates. Journal of Evolutionary Biology, 25(6), 1209-1215.
Kgope, B.S., W.J. Bond, and G.F. Midgley, 2010: Growth responses of African savanna
trees implicate atmospheric CO
2
as a driver of past and current changes in
savanna tree cover. Austral Ecology, 35(4), 451-463.
Kharuk, V.I., K.J. Ranson, P.A. Oskorbin, S.T. Im, and M.L. Dvinskaya, 2013: Climate
induced birch mortality in Trans-Baikal lake region, Siberia. Forest Ecology and
Management, 289, 385-392.
Kherchouche, D., M. Kalla, E.M. Gutiérrez, S. Attalah, and M. Bouzghaia, 2012:
Impact of droughts on Cedrus atlantica forests dieback in the Aurès (Algeria).
Journal of Life Sciences, 6, 1262-1269.
Khoury, C., B. Laliberte, and L. Guarino, 2010: Trends in ex situ conservation of plant
genetic resources: a review of global crop and regional conservation strategies.
Genetic Resources and Crop Evolution, 57(4), 625-639.
Kiesecker, J.M., 2011: Global stressors and the global decline of amphibians: tipping
the stress immunocompetency axis. Ecological Research, 26(5), 897-908.
Kimball, S., A.L. Angert, T.E. Huxman, and D.L. Venable, 2010: Contemporary climate
change in the Sonoran Desert favors cold-adapted species. Global Change
Biology, 16(5), 1555-1565.
Kindermann, G.E., I. McCallum, S. Fritz, and M. Obersteiner, 2008: A global forest
growing stock, biomass and carbon map based on FAO statistics. Silva Fennica,
42(3), 387-396.
Kinlan, B.P. and S.D. Gaines, 2003: Propagule dispersal in marine and terrestrial
environments: a community perspective. Ecology, 84(8), 2007-2020.
Kint, V., W. Aertsen, M. Campioli, D. Vansteenkiste, A. Delcloo, and B. Muys, 2012:
Radial growth change of temperate tree species in response to altered regional
climate and air quality in the period 1901-2008. Climatic Change, 115(2), 343-
363.
Kirdyanov, A.V., F. Hagedorn, A.A. Knorre, E.V. Fedotova, E.A. Vaganov, M.M.
Naurzbaev, P.A. Moiseev, and A. Rigling, 2012: 20
th
century tree-line advance
and vegetation changes along an altitudinal transect in the Putorana Mountains,
northern Siberia. Boreas, 41(1), 56-67.
Kirilenko, A.P. and R.A. Sedjo, 2007: Climate change impacts on forestry. Proceedings
of the National Academy of Sciences of the United States of America, 104(50),
19697-19702.
Kirschbaum, M.U.F., S. Saggar, K.R. Tate, D.L. Giltrap, A.-G.E. Ausseil, S. Greenhalgh,
and D. Whitehead, 2012: Comprehensive evaluation of the climate-change
implications of shifting land use between forest and grassland: New Zealand
as a case study. Agriculture, Ecosystems & Environment, 150, 123-138.
Kirwan, M.L. and L.K. Blum, 2011: Enhanced decomposition offsets enhanced
productivity and soil carbon accumulation in coastal wetlands responding to
climate change. Biogeosciences, 8(4), 987-993.
Kjøhl, M., A. Nielsen, and N.C. Stenseth, 2011: Potential Effects of Climate Change
on Crop Pollination. Food and Agriculture Organization of the United Nations
(FAO), Rome, Italy, 38 pp.
Klanderud, K. and O. Totland, 2005: Simulated climate change altered dominance
hierarchies and diversity of an alpine biodiversity hotspot. Ecology, 86(8),
2047-2054.
Klausmeyer, K.R. and M.R. Shaw, 2009: Climate change, habitat loss, protected areas
and the climate adaptation potential of species in Mediterranean ecosystems
worldwide. PLoS One, 4(7), e6392, doi:10.1371/journal.pone.0006392.
Klein Goldewijk, K., A. Beusen, and P. Janssen, 2010: Long term dynamic modeling
of global population and built-up area in a spatially explicit way: HYDE 3.1.
The Holocene, 20(4), 565-573.
Klein Goldewijk, K., A. Beusen, G. van Drecht, and M. de Vos, 2011: The HYDE 3.1
spatially explicit database of human induced land use change over the past
12,000 years. Global Ecology and Biogeography, 20, 73-86.
Klingberg, J., M. Engardt, J. Uddling, P.E. Karlsson, and H. Pleijel, 2011: Ozone risk
for vegetation in the future climate of Europe based on stomatal ozone uptake
calculations. Tellus Series A: Dynamic Meteorology and Oceanography, 63(1),
174-187.

343
Terrestrial and Inland Water Systems Chapter 4
4
Klos, R.J., G.G. Wang, W.L. Bauerle, and J.R. Rieck, 2009: Drought impact on forest
growth and mortality in the southeast USA: an analysis using Forest Health
and Monitoring data. Ecological Applications, 19(3), 699-708.
Knapp, A.K., J.M. Briggs, S.L. Collins, S.R. Archer, M.S. Bret-Harte, B.E. Ewers, and D.P.
Peters, 2007: Shrub encroachment in North American grasslands: shifts in
growth form dominance rapidly alters control of ecosystem carbon inputs.
Global Change Biology, 14(3), 615-623.
Knapp, A.K., C. Beier, D.D. Briske, A.T. Classen, Y. Luo, M. Reichstein, M.D. Smith, S.D.
Smith, J.E. Bell, P.A. Fay, J.L. Heisler, S.W. Leavitt, R. Sherry, B. Smith, and E. Weng,
2008: Consequences of more extreme precipitation regimes for terrestrial
ecosystems. Bioscience, 58(9), 811-821.
Knapp, S., I. Kühn, R. Wittig, W.A. Ozinga, P. Poschlod, and S. Klotz, 2008: Urbanization
causes shifts in species’ trait state frequencies. Preslia, 80(4), 375-388.
Knapp, S., L. Dinsmore, C. Fissore, S.E. Hobbie, I. Jakobsdottir, J. Kattge, J.R. King,
S. Klotz, J.P. McFadden, and J.M. Cavender-Bares, 2012: Phylogenetic and
functional characteristics of household yard floras and their changes along an
urbanization gradient. Ecology, 93(8 Suppl.), S83–S98.
Knohl, A. and D.D. Baldocchi, 2008: Effects of diffuse radiation on canopy gas
exchange processes in a forest ecosystem. Journal of Geophysical Research:
Biogeosciences, 113(G2), G02023, doi:10.1029/2007JG000663.
Knox, R., G. Bisht, J. Wang, and R. Bras, 2011: Precipitation variability over the forest-
to-nonforest transition in southwestern Amazonia. Journal of Climate, 24,
2368-2377.
Knudsen, E., A. Linden, C. Both, N. Jonzen, F. Pulido, N. Saino, W.J. Sutherland, L.A.
Bach, T. Coppack, T. Ergon, P. Gienapp, J.A. Gill, O. Gordo, A. Hedenstroom, E.
Lehikoinen, P.P. Marra, A.P. Moller, A.L.K. Nilsson, G. Peron, E. Ranta, D. Rubolini,
T.H. Sparks, F. Spina, C.E. Studds, S.A. Saether, P. Tryjanowski, and N.C. Stenseth,
2011: Challenging claims in the study of migratory birds and climate change.
Biological Reviews, 86(4), 928-946.
Knutti, R. and J. Sedláček, 2012: Robustness and uncertainties in the new CMIP5
climate model projections. Nature Climate Change, 3, 369-373.
Koehler, I.H., P.R. Poulton, K. Auerswald, and H. Schnyder, 2010: Intrinsic water-use
efficiency of temperate seminatural grassland has increased since 1857: an
analysis of carbon isotope discrimination of herbage from the Park Grass
Experiment. Global Change Biology, 16(5), 1531-1541.
Kollár, J., P. Hrubík, and S. Tkáčová, 2009: Monitoring of harmful insect species in
urban conditions in selected model areas of Slovakia. Plant Protection Science,
45, 119-124.
Kollberg, I., H. Bylund, A. Schmidt, J. Gershenzon, and C. Björkman, 2013: Multiple
effects of temperature, photoperiod and food quality on the performance of a
pine sawfly. Ecological Entomology, 38(2), 201-208.
Konarzewski, T.K., B.R. Murray, and R.C. Godfree, 2012: Rapid development of adaptive,
climate-driven clinal variation in seed mass in the invasive annual forb Echium
plantagineum L. PLoS One, 7(12), e49000, doi:10.1371/journal.pone.0049000.
Kongstad, J., I.K. Schmidt, T. Riis-Nielsen, M.F. Arndal, T.N. Mikkelsen, and C. Beier,
2012: High resilience in heathland plants to changes in temperature, drought,
and CO
2
in combination: results from the CLIMAITE Experiment. Ecosystems,
15(2), 269-283.
Körner, C. and D. Basler, 2010: Phenology under global warming. Science,
327(5972), 1461-1462.
Körner, C., J.A. Morgan, and R. Norby, 2007: CO
2
fertilisation: when, where, how
much? In: Terrestrial Ecosystems in a Changing World [Canadell, S.G., D.E.
Pataki, and L.F. Pitelka (eds.)]. Springer, Berlin Heidelberg, Germany, pp. 9-22.
Koutavas, A., 2008: Late 20
th
century growth acceleration in greek firs (Abies
cephalonica) from Cephalonia Island, Greece: a CO
2
fertilization effect?
Dendrochronologia, 26(1), 13-19.
Kovach-Orr, C. and G.F. Fussmann, 2013: Evolutionary and plastic rescue in
multitrophic model communities. Philosophical Transactions of the Royal
Society B, 368(1610), 20120084, doi:10.1098/rstb.2012.0084.
Koven, C.D., W.J. Riley, and A. Stern, 2013: Analysis of permafrost thermal dynamics
and response to climate change in the CMIP5 Earth System Models. Journal of
Climate, 26(6), 1877-1900.
Kraft, N.J.B., M.R. Metz, R.S. Condit, and J. Chave, 2010: The relationship between
wood density and mortality in a global tropical forest data set. New Phytologist,
188(4), 1124-1136.
Kramer, K., B. Degen, J. Buschbom, T. Hickler, W. Thuiller, M.T. Sykes, and W. de Winter,
2010: Modelling exploration of the future of European beech (Fagus sylvatica
L.) under climate change – range, abundance, genetic diversity and adaptive
response. Forest Ecology and Management, 259(11), 2213-2222.
Kramer, K., R.J. Bijlsma, T. Hickler, and W. Thuiller, 2012: Why would plant species
become extinct locally if growing conditions improve? International Journal of
Biological Sciences, 8(8), 1121-1129.
Kremer, A., O. Ronce, J.J. Robledo-Arnuncio, F. Guillaume, G. Bohrer, R. Nathan, J.R.
Bridle, R. Gomulkiewicz, E.K. Klein, K. Ritland, A. Kuparinen, S. Gerber, and S.
Schueler, 2012: Long-distance gene flow and adaptation of forest trees to rapid
climate change. Ecology Letters, 15(4), 378-392.
Kriegler, E., J.W. Hall, H. Held, R. Dawson, and H.J. Schellnhuber, 2009: Imprecise
probability assessment of tipping points in the climate system. Proceedings of
the National Academy of Sciences of the United States of America, 106(13),
5041-5046.
Kropelin, S., D. Verschuren, A.M. Lezine, H. Eggermont, C. Cocquyt, P. Francus, J.P.
Cazet, M. Fagot, B. Rumes, J.M. Russell, F. Darius, D.J. Conley, M. Schuster, H.
von Suchodoletz, and D.R. Engstrom, 2008: Climate-driven ecosystem succession
in the Sahara: the past 6000 years. Science, 320(5877), 765-768.
Kudo, G., Y. Amagai, B. Hoshino, and M. Kaneko, 2011: Invasion of dwarf bamboo
into alpine snow-meadows in northern Japan: pattern of expansion and impact
on species diversity. Ecology and Evolution, 1(1), 85-96.
Kuhlmann, M., D. Guo, R. Veldtman, and J. Donaldson, 2012: Consequences of warming
up a hotspot: species range shifts within a centre of bee diversity. Diversity and
Distributions, 18(9), 885-897.
Kukowski, K., S. Schwinning, and B. Schwartz, 2013: Hydraulic responses to extreme
drought conditions in three co-dominant tree species in shallow soil over
bedrock. Oecologia, 171(4), 819-830.
Kuldna, P., K. Peterson, H. Poltimae, and J. Luig, 2009: An application of DPSIR framework
to identify issues of pollinator loss. Ecological Economics, 69(1), 32-42.
Kullman, L. and L. Öberg, 2009: Post-Little Ice Age tree line rise and climate warming
in the Swedish Scandes: a landscape ecological perspective. Journal of Ecology,
97(3), 415-429.
Kundzewicz, Z.W., L.J. Mata, N.W. Arnell, P. Döll, P. Kabat, B. Jiménez, K.A. Miller, T.
Oki, Z. Sen, and I.A. Shiklomanov, 2007: Freshwater resources and their
management. In: Climate Change 2007: Impacts, Adaptation and Vulnerability.
Contribution of Working Group II to the Fourth Assessment Report of the
Intergovernmental Panel on Climate Change [Parry, M.L., O.F. Canziani, J.P.
Palutikof, P.J. van der Linden, and C.E. Hanson (eds.)]. Cambridge University
Press, Cambridge, UK and New York, NY, USA, pp. 173-210.
Kuparinen, A., O. Savolainen, and F.M. Schurr, 2010: Increased mortality can promote
evolutionary adaptation of forest trees to climate change. Forest Ecology and
Management, 259(5), 1003-1008.
Kurz, W.A., C.C. Dymond, G. Stinson, G.J. Rampley, E.T. Neilson, A.L. Carroll, T. Ebata,
and L. Safranyik, 2008: Mountain pine beetle and forest carbon feedback to
climate change. Nature, 452(7190), 987-990.
Kusano, T. and M. Inoue, 2008: Long-term trends toward earlier breeding of Japanese
amphibians. Journal of Herpetology, 42(4), 608-614.
Kuussaari, M., R. Bommarco, R.K. Heikkinen, A. Helm, J. Krauss, R. Lindborg, E.
Ockinger, M. Partel, J. Pino, F. Roda, C. Stefanescu, T. Teder, M. Zobel, and I. Steffan-
Dewenter, 2009: Extinction debt: a challenge for biodiversity conservation.
Trends in Ecology & Evolution, 24(10), 564-571.
Kvalevåg, M.M. and G. Myhre, 2007: Human impact on direct and diffuse solar
radiation during the industrial era. Journal of Climate, 20(19), 4874-4883.
Lambert, A.M., A.J. Miller-Rushing, and D.W. Inouye, 2010: Changes in snowmelt
date and summer precipitation affect the flowering phenology of Erythronium
grandiflorum (Glacier Lily; Liliaceae). American Journal of Botany, 97(9), 1431-
1437.
Lambin, E.F. and P. Meyfroidt, 2011: Global land use change, economic globalization,
and the looming land scarcity. Proceedings of the National Academy of Sciences
of the United States of America, 108(9), 3465-3472.
Lane, J.E., L.E.B. Kruuk, A. Charmantier, J.O. Murie, and F.S. Dobson, 2012: Delayed
phenology and reduced fitness associated with climate change in a wild
hibernator. Nature, 489(7417), 554-557.
Langan, S.J., L. Johnston, M.J. Donaghy, A.F. Youngson, D.W. Hay, and C. Soulsby,
2001: Variation in river water temperatures in an upland stream over a 30-year
period. Science of the Total Environment, 265(1-3), 195-207.
Langley, J.A. and J.P. Megonigal, 2010: Ecosystem response to elevated CO
2
levels
limited by nitrogen-induced plant species shift. Nature, 466(7302), 96-99.
Lantz, T.C., S.V. Kokelj, S.E. Gergel, and G.H.R. Henry, 2009: Relative impacts of
disturbance and temperature: persistent changes in microenvironment and
vegetation in retrogressive thaw slumps. Global Change Biology, 15(7), 1664-
1675.

344
Chapter 4 Terrestrial and Inland Water Systems
4
Lantz, T.C., S.E. Gergel, and G.H.R. Henry, 2010: Response of green alder (Alnus viridis
subsp. fruticosa) patch dynamics and plant community composition to fire and
regional temperature in north-western Canada. Journal of Biogeography, 37(8),
1597-1610.
Lapola, D.M., M.D. Oyama, and C.A. Nobre, 2009: Exploring the range of climate
biome projections for tropical South America: the role of CO
2
fertilization and
seasonality. Global Biogeochemical Cycles, 23(3), GB3003, doi:10.1029/
2008GB003357.
Lapola, D.M., R. Schaldach, J. Alcamo, A. Bondeau, J. Koch, C. Koelking, and J.A. Priess,
2010: Indirect land-use changes can overcome carbon savings from biofuels
in Brazil. Proceedings of the National Academy of Sciences of the United States
of America, 107(8), 3388-3393.
Larsen, K.S., L.C. Andresen, C. Beier, S. Jonasson, K.R. Albert, P. Ambus, M.F. Arndal,
M.S. Carter, S. Christensen, M. Holmstrup, A. Ibrom, J. Kongstad, L. van der
Linden, K. Maraldo, A. Michelsen, T.N. Mikkelsen, K. Pilegaard, A. Prieme, H. Ro-
Poulsen, I.K. Schmidt, M.B. Selsted, and K. Stevnbak, 2011: Reduced N cycling
in response to elevated CO
2
, warming, and drought in a Danish heathland:
synthesizing results of the CLIMAITE project after two years of treatments.
Global Change Biology, 17(5), 1884-1899.
Laurance, W.F., D.C. Useche, L.P. Shoo, S.K. Herzog, M. Kessler, F. Escobar, G. Brehm,
J.C. Axmacher, I.C. Chen, L.A. Gamez, P. Hietz, K. Fiedler, T. Pyrcz, J. Wolf, C.L.
Merkord, C. Cardelus, A.R. Marshall, C. Ah-Peng, G.H. Aplet, M.D. Arizmendi,
W.J. Baker, J. Barone, C.A. Bruhl, R.W. Bussmann, D. Cicuzza, G. Eilu, M.E. Favila,
A. Hemp, C. Hemp, J. Homeier, J. Hurtado, J. Jankowski, G. Kattan, J. Kluge, T.
Kromer, D.C. Lees, M. Lehnert, J.T. Longino, J. Lovett, P.H. Martin, B.D. Patterson,
R.G. Pearson, K.S.H. Peh, B. Richardson, M. Richardson, M.J. Samways, F.
Senbeta, T.B. Smith, T.M.A. Utteridge, J.E. Watkins, R. Wilson, S.E. Williams, and
C.D. Thomas, 2011: Global warming, elevational ranges and the vulnerability
of tropical biota. Biological Conservation, 144(1), 548-557.
Lavergne, S., N. Mouquet, W. Thuiller, and O. Ronce, 2010: Biodiversity and climate
change: integrating evolutionary and ecological responses of species and
communities. Annual Review of Ecology, Evolution, and Systematics, 41, 321-350.
Lavergne, S., M.E.K. Evans, I.J. Burfield, F. Jiguet, and Thuiller.W., 2013: Are species’
responses to global change predicted by past niche evolution? Philosophical
Transactions of the Royal Society B, 368(1610), 20120091, doi:10.1098/
rstb.2012.0091.
Lawrence, D.M. and S.C. Swenson, 2011: Permafrost response to increasing Arctic
shrub abundance depends on the relative influence of shrubs on local soil cool-
ing versus large-scale climate warming. Environmental Research Letters, 6(4),
045504, doi:10.1088/1748-9326/6/4/045504.
Lawrence, D.M., P.E. Thornton, K.W. Oleson, and G.B. Bonan, 2007: The partitioning
of evpotranspiration into transpiration, soil evaporation, and canopy evaporation
in a GCM: impacts on land-atmosphere interaction. Journal of Hydrometeorology,
8, 862-880.
Lawson, C.R., J.J. Bennie, C.D. Thomas, J.A. Hodgson, and R.J. Wilson, 2012: Local and
landscape management of an expanding range margin under climate change.
Journal of Applied Ecology, 49, 552-561.
Le Conte, Y. and M. Navajas, 2008: Climate change: impact on honey bee populations
and diseases. Revue Scientifique Et Technique – Office International Des
Epizooties, 27(2), 499-510.
Le Quéré, C., M.R. Raupach, J.G. Canadell, and G. Marland, 2009: Trends in the
sources and sinks of carbon dioxide. Nature Geoscience, 2(12), 831-836.
Le Quéré, C., R.J. Andres, T. Boden, T. Conway, R.A. Houghton, J.I. House, G. Marland,
G.P. Peters, G. van der Werf, A. Ahlström, R.M. Andrew, L. Bopp, J.G. Canadell, P.
Ciais, S.C. Doney, C. Enright, P. Friedlingstein, C. Huntingford, A.K. Jain, C.
Jourdain, E. Kato, R.F. Keeling, K. Klein Goldewijk, S. Levis, P. Levy, M. Lomas, B.
Poulter, M.R. Raupach, J. Schwinger, S. Sitch, B.D. Stocker, N. Viovy, S. Zaehle,
and N. Zeng, 2012: The global carbon budget 1959-2011. Earth System Science
Data, 5(2), 1107-1157.
Leadley, P., H.N. Pereira, R. Alkemade, J.F. Fernandez-Manjarrés, V. Proença, J.P.W.
Scharlemann, and M.J. Walpole, 2010: Biodiversity Scenarios: Projections of 21
st
Century Change in Biodiversity and Associated Ecosystem Services. A Technical
Report for the Global Biodiversity Outlook 3, Technical Series No. 50, Secretariat
of the Convention on Biological Diversity, Montreal, Canada, 132 pp.
Leakey, A.D.B., E.A. Ainsworth, C.J. Bernacchi, A. Rogers, S.P. Long, and D.R. Ort, 2009:
Elevated CO
2
effects on plant carbon, nitrogen, and water relations: six important
lessons from FACE. Journal of Experimental Botany, 60(10), 2859-2876.
Leal, M. and A.R. Gunderson, 2012: Rapid change in the thermal tolerance of a
tropical lizard. American Naturalist, 180(6), 815-822.
Ledger, M.E., L.E. Brown, F.K. Edwards, A.M. Milner, and G. Woodward, 2013: Drought
alters the structure and functioning of complex food webs. Nature Climate
Change, 3(3), 223-227.
Lee, M., P. Manning, J. Rist, S.A. Power, and C. Marsh, 2010: A global comparison of
grassland biomass responses to CO
2
and nitrogen enrichment. Philosophical
Transactions of the Royal Society B, 365(1549), 2047-2056.
Leishman, M.R., T. Haslehurst, A. Ares, and Z. Baruch, 2007: Leaf trait relationships
of native and invasive plants: community- and global-scale comparisons. New
Phytologist, 176(3), 635-643.
Lenihan, J.M., D. Bachelet, R.P. Neilson, and R. Drapek, 2008: Response of vegetation
distribution, ecosystem productivity, and fire to climate change scenarios for
California. Climatic Change, 87, S215-S230.
Lenoir, J., J.C. Gegout, P.A. Marquet, P. de Ruffray, and H. Brisse, 2008: A significant
upward shift in plant species optimum elevation during the 20
t
h
century.
Science, 320(5884), 1768-1771.
Lenoir, J., J.C. Gegout, J.L. Dupouey, D. Bert, and J.C. Svenning, 2010: Forest plant
community changes during 1989-2007 in response to climate warming in the
Jura Mountains (France and Switzerland). Journal of Vegetation Science, 21(5),
949-964.
Lenton, T.M., H. Held, E. Kriegler, J.W. Hall, W. Lucht, S. Rahmstorf, and H.J. Schellnhuber,
2008: Tipping elements in the Earth’s climate system. Proceedings of the
National Academy of Sciences of the United States of America, 105(6), 1786-
1793.
Leonelli, G., M. Pelfini, U. Morra di Cella, and V. Garavaglia, 2011: Climate warming
and the recent treeline shift in the European Alps: the role of geomorphological
factors in high-altitude sites. AMBIO: A Journal of the Human Environment,
40(3), 264-273.
Lermen, D., B. Blomeke, R. Browne, A. Clarke, P.W. Dyce, T. Fixemer, G.R. Fuhr, W.V.
Holt, K. Jewgenow, R.E. Lloyd, S. Lotters, M. Paulus, G.M. Reid, D.H. Rapoport,
D. Rawson, J. Ringleb, O.A. Ryder, G. Sporl, T. Schmitt, M. Veith, and P. Muller,
2009: Cryobanking of viable biomaterials: implementation of new strategies
for conservation purposes. Molecular Ecology, 18(6), 1030-1033.
Leuning, R., 1995: A critical appraisal of a combined stomatalphotosynthesis model
for C
3
plants. Plant, Cell and Environment, 18, 339-355.
Leuzinger, S. and C. Körner, 2010: Rainfall distribution is the main driver of runoff
under future CO
2
-concentration in a temperate deciduous forest. Global Change
Biology, 16(1), 246-254.
Leuzinger, S., Y.Q. Luo, C. Beier, W. Dieleman, S. Vicca, and C. Korner, 2011: Do global
change experiments overestimate impacts on terrestrial ecosystems? Trends in
Ecology & Evolution, 26(5), 236-241.
Levis, S., 2010: Modeling vegetation and land use in models of the Earth System.
Wiley Interdisciplinary Reviews: Climate Change, 1(6), 840-856.
Lewis, S.L., J. Lloyd, S. Sitch, E.T.A. Mitchard, and W.F. Laurance, 2009a: Changing
ecology of tropical forests: evidence and drivers. Annual Review of Ecology
Evolution and Systematics, 40, 529-549.
Lewis, S.L., G. Lopez-Gonzalez, B. Sonke, K. Affum-Baffoe, T.R. Baker, L.O. Ojo, O.L.
Phillips, J.M. Reitsma, L. White, J.A. Comiskey, M.N. Djuikouo, C.E.N. Ewango,
T.R. Feldpausch, A.C. Hamilton, M. Gloor, T. Hart, A. Hladik, J. Lloyd, J.C. Lovett,
J.R. Makana, Y. Malhi, F.M. Mbago, H.J. Ndangalasi, J. Peacock, K.S.H. Peh, D.
Sheil, T. Sunderland, M.D. Swaine, J. Taplin, D. Taylor, S.C. Thomas, R. Votere, and
H. Wöll, 2009b: Increasing carbon storage in intact African tropical forests.
Nature, 457, 1003-1007.
Lewis, S.L., P.M. Brando, O.L. Phillips, G.M.F. van der Heijden, and D. Nepstad, 2011:
The 2010 Amazon drought. Science, 331(6017), 554.
Li, D.Z. and H.W. Pritchard, 2009: The science and economics of ex situ plant
conservation. Trends in Plant Science, 14(11), 614-621.
Li, W.H., R.E. Dickinson, R. Fu, G.Y. Niu, Z.L. Yang, and J.G. Canadell, 2007: Future
precipitation changes and their implications for tropical peatlands. Geophysical
Research Letters, 34, L01403, doi:10.1029/2006GL028364.
Li, Z., W.-z. Liu, X.-c. Zhang, and F.-l. Zheng, 2009: Impacts of land use change and
climate variability on hydrology in an agricultural catchment on the Loess
Plateau of China. Journal of Hydrology, 377(1-2), 35-42.
Liao, J.D., T.W. Boutton, and J.D. Jastrow, 2006: Storage and dynamics of carbon and
nitrogen in soil physical fractions following woody plant invasion of grassland.
Soil Biology and Biochemistry, 38(11), 3184-3196.
Liljedahl, A., L. Hinzman, R. Busey, and K. Yoshikawa, 2007: Physical short-term
changes after a tussock tundra fire, Seward Peninsula, Alaska. Journal of
Geophysical Research: Earth Surface, 112(F2), F02S07, doi:10.1029/
2006JF000554.

345
Terrestrial and Inland Water Systems Chapter 4
4
Limpens, J., F. Berendse, C. Blodau, J.G. Canadell, C. Freeman, J. Holden, N. Roulet, H.
Rydin, and G. Schaepman-Strub, 2008: Peatlands and the carbon cycle: from
local processes to global implications – a synthesis. Biogeosciences, 5(5), 1475-
1491.
Limpens, J., G. Granath, U. Gunnarsson, R. Aerts, S. Bayley, L. Bragazza, J. Bubier, A.
Buttler, L.J.L. van den Berg, A.J. Francez, R. Gerdol, P. Grosvernier, M. Heijmans,
M.R. Hoosbeek, S. Hotes, M. Ilomets, I. Leith, E.A.D. Mitchell, T. Moore, M.B.
Nilsson, J.F. Nordbakken, L. Rochefort, H. Rydin, L.J. Sheppard, M. Thormann,
M.M. Wiedermann, B.L. Williams, and B. Xu, 2011: Climatic modifiers of the
response to nitrogen deposition in peat-forming Sphagnum mosses: a meta-
analysis. New Phytologist, 191(2), 496-507.
Linares, J.C., J.J. Camarero, and J.A. Carreira, 2009: Interacting effects of changes in
climate and forest cover on mortality and growth of the southernmost
European fir forests. Global Ecology and Biogeography, 18(4), 485-497.
Linares, J.C, P.A. Tíscar, J.J. Camarero, L. Taïqui, and B. Viñegla, 2011: Tree growth
decline on relict western-Mediterrenean mountain forests: causes and impacts.
In: Forest Decline: Causes and Impacts [Jenkins, J.A.(ed)]. Nova Publishers, New
York, NY, USA, pp. 91-110.
Lindner, M., M. Maroschek, S. Netherer, A. Kremer, A. Barbati, J. Garcia-Gonzalo, R.
Seidl, S. Delzon, P. Corona, M. Kolstrom, M.J. Lexer, and M. Marchetti, 2010:
Climate change impacts, adaptive capacity, and vulnerability of European forest
ecosystems. Forest Ecology and Management, 259(4), 698-709.
Lindroth, R.L., 2010: Impacts of elevated atmospheric CO
2
and O
3
on forests:
phytochemistry, trophic interactions, and ecosystem dynamics. Journal of
Chemical Ecology, 36(1), 2-21.
Lips, K.R., J. Diffendorfer, J.R. Mendelson, and M.W. Sears, 2008: Riding the wave:
reconciling the roles of disease and climate change in amphibian declines. PLoS
Biology, 6(3), 441-454.
Littell, J.S., D. McKenzie, D.L. Peterson, and A.L. Westerling, 2009: Climate and wildfire
area burned in western U.S. ecoprovinces, 1916-2003. Ecological Applications,
19, 1003-1021.
Littell, J.S., E.E. Oneil, D. McKenzie, J.A. Hicke, J.A. Lutz, R.A. Norheim, and M.M. Elsner,
2010: Forest ecosystems, disturbance, and climatic change in Washington State,
USA. Climatic Change, 102(1-2), 129-158.
Liu, H., A. Park Williams, C. Allen, D. Guo, X. Wu, O.A. Anenkhonov, E. Liang, D.V.
Sandanov, Y. Yin, Z. Qi, and N.K. Badmaeva, 2013: Rapid warming accelerates
tree growth decline in semi-arid forests of Inner Asia. Global Change Biology,
19(8), 2500-2510.
Liu, W., Z. Zhang, and S. Wan, 2009: Predominant role of water in regulating soil and
microbial respiration and their responses to climate change in a semiarid
grassland. Global Change Biology, 15(1), 184-195.
Livingstone, D.M. and R. Adrian, 2009: Modeling the duration of intermittent ice
cover on a lake for climate-change studies. Limnology and Oceanography,
54(5), 1709-1722.
Lloyd, A.H. and C.L. Fastie, 2003: Recent changes in tree line forest distribution and
structure in interior Alaska. Ecoscience, 10, 176-185.
Lloyd, A.H., A.G. Bunn, and L. Berner, 2011: A latitudinal gradient in tree growth
response to climate warming in the Siberian taiga. Global Change Biology,
17(5), 1935-1945.
Lloyd, J. and G.D. Farquhar, 2008: Effects of rising temperatures and CO
2
on the
physiology of tropical forest trees. Philosophical Transactions of the Royal
Society B, 363(1498), 1811-1817.
Loader, N.J., R.P.D. Walsh, I. Robertson, K. Bidin, R.C. Ong, G. Reynolds, D. McCarroll,
M. Gagen, and G.H.F. Young, 2011: Recent trends in the intrinsic water-use
efficiency of ringless rainforest trees in Borneo. Philosophical Transactions of
the Royal Society B, 366(1582), 3330-3339.
Loarie, S.R., P.B. Duffy, H. Hamilton, G.P. Asner, C.B. Field, and D.D. Ackerly, 2009: The
velocity of climate change. Nature, 462, 1052-1055.
Loarie, S.R., D.B. Lobell, G.P. Asner, Q. Mu, and C.B. Field, 2011: Direct impacts on
local climate of sugar-cane expansion in Brazil. Nature Climate Change, 1(2),
105-109.
Lobell, D.B., W. Schlenker, and J. Costa-Roberts, 2011: Climate trends and global crop
production since 1980. Science, 333(6042), 616-620.
Long, S.P., E.A. Ainsworth, A. Rogers, and D.R. Ort, 2004: Rising atmospheric carbon
dioxied: plants FACE the future. Annual Review of Plant Biology, 55(1), 591-
628.
Longobardi, P., A. Montenegro, H. Beltrami, and M. Eby, 2012: Spatial scale
dependency of the modelled climatic response to deforestation. Biogeosciences
Discussions, 9, 14639-14687.
Lopez-Vaamonde, C., D. Agassiz, S. Augustin, J. De Prins, W. De Prins, S. Gomboc, P.
Ivinskis, O. Karsholt, A. Koutroumpas, F. Koutroumpa, Z. Laštůvka, E. Marabuto,
E. Olivella, L. Przybylowicz, A. Roques, N. Ryrholm, H. Šefrová, P. Šima, I. Sims,
S. Sinev, B. Skulev, R. Tomov, A. Zilli, and D. Lees, 2010: Lepidoptera. In: Biorisk,
Vol. 4, Part 2: Alien Terrestrial Arthropods of Europe [Roques, A., M. Kenis, D.
Lees, C. Lopez-Vaamonde, W. Rabitsch, J.-Y. Rasplus, and D.B. Roy (eds.)]. Pensoft
Publishers, Sofia, Bulgaria, pp. 603-668.
Loreau, M., N. Mouquet, and R.D. Holt, 2003: Meta-ecosystems: a theoretical
framework for a spatial ecosystem ecology. Ecology Letters, 6(8), 673-679.
Lorenzen, E.D., D. Nogues-Bravo, L. Orlando, J. Weinstock, J. Binladen, K.A. Marske, A.
Ugan, M.K. Borregaard, M.T.P. Gilbert, R. Nielsen, S.Y.W. Ho, T. Goebel, K.E. Graf,
D. Byers, J.T. Stenderup, M. Rasmussen, P.F. Campos, J.A. Leonard, K.P. Koepfli, D.
Froese, G. Zazula, T.W. Stafford, K. Aaris-Sorensen, P. Batra, A.M. Haywood, J.S.
Singarayer, P.J. Valdes, G. Boeskorov, J.A. Burns, S.P. Davydov, J. Haile, D.L. Jenkins,
P. Kosintsev, T. Kuznetsova, X.L. Lai, L.D. Martin, H.G. McDonald, D. Mol, M.
Meldgaard, K. Munch, E. Stephan, M. Sablin, R.S. Sommer, T. Sipko, E. Scott, M.A.
Suchard, A. Tikhonov, R. Willerslev, R.K. Wayne, A. Cooper, M. Hofreiter, A. Sher,
B. Shapiro, C. Rahbek, and E. Willerslev, 2011: Species-specific responses of Late
Quaternary megafauna to climate and humans. Nature, 479, 359-364.
Loss, S.R., L.A. Terwilliger, and A.C. Peterson, 2011: Assisted colonization: integrating
conservation strategies in the face of climate change. Biological Conservation,
144(1), 92-100.
Loustau, D., 2010: Forests, Carbon Cycle and Climate Change. Éditions Quae,
Versailles, France, 350 pp.
Lu, C.Q., H.Q. Tian, M.L. Liu, W. Ren, X.F. Xu, G.S. Chen, and C. Zhang, 2012: Effect of
nitrogen deposition on China’s terrestrial carbon uptake in the context of
multifactor environmental changes. Ecological Applications, 22(1), 53-75.
Lu, J., C. Deser, and T. Reichler, 2009: Cause of the widening of the tropical belt since
1958. Geophysical Research Letters, 36, L03803, doi:10.1029/2008GL036076.
Luckman, B. and T. Kavanagh, 2000: Impact of climate fluctuations on mountain
environments in the Canadian Rockies. AMBIO: A Journal of the Human
Environment, 29(7), 371-380.
Lunt, I.D., L.M. Winsemius, S.P. McDonald, J.W. Morgan, and R.L. Dehaan, 2010: How
widespread is woody plant encroachment in temperate Australia? Changes in
woody vegetation cover in lowland woodland and coastal ecosystems in
Victoria from 1989 to 2005. Journal of Biogeography, 37(4), 722-732.
Luo, Y.Q., 2007: Terrestrial carbon-cycle feedback to climate warming. Annual Review
of Ecology Evolution and Systematics, 38, 683-712.
Luo, Y.Q., D. Gerten, G. Le Maire, W.J. Parton, E.S. Weng, X.H. Zhou, C. Keough, C.
Beier, P. Ciais, W. Cramer, J.S. Dukes, B. Emmett, P.J. Hanson, A. Knapp, S. Linder,
D. Nepstad, and L. Rustad, 2008: Modeled interactive effects of precipitation,
temperature, and CO
2
on ecosystem carbon and water dynamics in different
climatic zones. Global Change Biology, 14(9), 1986-1999.
Luyssaert, S., P. Ciais, S.L. Piao, E.D. Schulze, M. Jung, S. Zaehle, M.J. Schelhaas, M.
Reichstein, G. Churkina, D. Papale, G. Abril, C. Beer, J. Grace, D. Loustau, G.
Matteucci, F. Marnani, G.J. Nabuurs, H. Verbeeck, M. Sulkava, G.R. Van Der Werf,
I.A. Janssens, and Members of the CarboEurope-IP Synthesis Team, 2010: The
European carbon balance. 3: forests. Global Change Biology, 16(5), 1429-1450.
Lydeard, C., R.H. Cowie, W.F. Ponder, A.E. Bogan, P. Bouchet, S.A. Clark, K.S.
Cummings, T.J. Frest, O. Gargominy, D.G. Herbert, R. Hershler, K.E. Perez, B. Roth,
M. Seddon, E.E. Strong, and F.G. Thompson, 2004: The global decline of non-
marine mollusks. BioScience, 54(4), 321-330.
Ma, L.N., X.T. Lu, Y. Liu, J.X. Guo, N.Y. Zhang, J.Q. Yang, and R.Z. Wang, 2011: The ef-
fects of warming and nitrogen addition on soil nitrogen cycling in a temperate
grassland, Northeastern China. PLoS One, 6(11), e27645, doi:10.1371/
journal.pone.0027645.
Ma, T. and C.G. Zhou, 2012: Climate-associated changes in spring plant phenology
in China. International Journal of Biometeorology, 56(2), 269-275.
Ma, Z., C. Peng, Q. Zhu, H. Chen, G. Yu, W.H. Li, X. Zhou, W. Wang, and W. Zhang,
2012: Regional drought-induced reduction in the biomass carbon sink of
Canada’s boreal forests. Proceedings of the National Academy of Sciences of
the United States of America, 109(7), 2423-2427.
MacDonald, G.M., 2010: Global warming and the Arctic: a new world beyond the
reach of the Grinnellian niche? The Journal of Experimental Biology, 213, 855-
861.
MacDonald, G.M., K.D. Bennett, S.T. Jackson, L. Parducci, F.A. Smith, J.P. Smol, and
K.J. Willis, 2008: Impacts of climate change on species, populations and
communities: palaeobiogeographical insights and frontiers. Progress in Physical
Geography, 32(2), 139-172.

346
Chapter 4 Terrestrial and Inland Water Systems
4
Macedo, M.N., R.S. DeFries, D.C. Morton, C.M. Stickler, G.L. Galford, and Y.E.
Shimabukuro, 2012: Decoupling of deforestation and soy production in the
southern Amazon during the late 2000s. Proceedings of the National Academy
of Sciences of the United States of America, 109(4), 1341-1346.
Macias Fauria, M. and E.A. Johnson, 2008: Climate and wildfires in the North American
boreal forest. Philosophical Transactions of the Royal Society B, 363(1501),
2317-2329.
Mack, M.C., K.K. Treseder, K.L. Manies, J.W. Harden, E.A.G. Schuur, J.G. Vogel, J.T.
Randerson, and F.S. Chapin, 2008: Recovery of aboveground plant biomass and
productivity after fire in mesic and dry black spruce forests of interior Alaska.
Ecosystems, 11(2), 209-225.
Mack, M.C., M.S. Bret-Harte, T.N. Hollingsworth, R.R. Jandt, E.A.G. Schuur, G.R. Shaver,
and D.L. Verbyla, 2011: Carbon loss from an unprecedented Arctic tundra
wildfire. Nature, 475(7357), 489-492.
Mackelprang, R., M.P. Waldrop, K.M. DeAngelis, M.M. David, K.L. Chavarria, S.J.
Blazewicz, E.M. Rubin, and J.K. Jansson, 2011: Metagenomic analysis of a
permafrost microbial community reveals a rapid response to thaw. Nature,
480(7377), 368-371.
Maclachlan, J.S., J.J. Hellmann, and M.W. Schwarz, 2007: A framework for debate of
assisted migration in an era of climate change. Conservation Biology, 21(2),
297-302.
Magalhães, M.F., P. Beja, I.J. Schlosser, and M.J. Collares-Pereira, 2007: Effects of
multi-year droughts on fish assemblages of seasonally drying Mediterranean
streams. Freshwater Biology, 52(8), 1494-1510.
Magnani, F., M. Mencuccini, M. Borghetti, P. Berbigier, F. Berninger, S. Delzon, A. Grelle,
P. Hari, P.G. Jarvis, P. Kolari, A.S. Kowalski, H. Lankreijer, B.E. Law, A. Lindroth, D.
Loustau, G. Manca, J.B. Moncrieff, M. Rayment, V. Tedeschi, R. Valentini, and J.
Grace, 2007: The human footprint in the carbon cycle of temperate and boreal
forests. Nature, 447(7146), 848-850.
Mainka, S.A. and G.W. Howard, 2010: Climate change and invasive species: double
jeopardy. Integrative Zoology, 5(2), 102-111.
Maiorano, L., A. Falcucci, N.E. Zimmermann, A. Psomas, J. Pottier, D. Baisero, C.
Rondinini, A. Guisan, and L. Boitani, 2011: The future of terrestrial mammals in
the Mediterranean basin under climate change. Philosophical Transactions of
the Royal Society B, 366(1578), 2681-2692.
Malcolm, J.R., C.R. Liu, R.P. Neilson, L. Hansen, and L. Hannah, 2006: Global warming
and extinctions of endemic species from biodiversity hotspots. Conservation
Biology, 20(2), 538-548.
Malhi, Y., J.T. Roberts, R.A. Betts, T.J. Killeen, W. Li, and C.A. Nobre, 2008: Climate change,
deforestation, and the fate of the Amazon. Science, 319(5860), 169-172.
Malhi, Y., L.E.O.C. Aragao, D. Galbraith, C. Huntingford, R. Fisher, P. Zelazowski, S.
Sitch, C. McSweeney, and P. Meir, 2009a: Exploring the likelihood and mechanism
of a climate-change-induced dieback of the Amazon rainforest. Proceedings of
the National Academy of Sciences of the United States of America, 106(49),
20610-20615.
Malhi, Y., L. Aragao, D.B. Metcalfe, R. Paiva, C.A. Quesada, S. Almeida, L. Anderson, P.
Brando, J.Q. Chambers, A.C.L. da Costa, L.R. Hutyra, P. Oliveira, S. Patino, E.H.
Pyle, A.L. Robertson, and L.M. Teixeira, 2009b: Comprehensive assessment of
carbon productivity, allocation and storage in three Amazonian forests. Global
Change Biology, 15(5), 1255-1274.
Malmqvist, B., S.D. Rundle, A.P. Covich, A.G. Hildrew, C.T. Robinson, and C.R.
Townsend, 2008: Prospects for streams and rivers: an ecological perspective.
In: Aquatic Ecosystems: Trends and Global Prospects [Polunin, N.V.C. (ed.)].
Cambridge University Press, Cambridge, UK, pp. 19-29.
Maniatis, D., Y. Malhi, L.S. Andre, D. Mollicone, N. Barbier, S. Saatchi, M. Henry, L.
Tellier, M. Schwartzenberg, and M. White, 2011: Evaluating the potential of
commercial forest inventory data to report on forest carbon stock and forest
carbon stock changes for REDD+ under the UNFCCC. International Journal of
Forestry Research, 2011, 134526, doi:10.1155/2011/134526.
Mann, D.H., T.S. Rupp, M.A. Olson, and P.A. Duffy, 2012: Is Alaska’s boreal forest now
crossing a major ecological threshold? Arctic Antarctic and Alpine Research,
44(3), 319-331.
Maraldo, K., I.K. Schmidt, C. Beier, and M. Holmstrup, 2008: Can field populations
of the enchytraeid, Cognettia sphagnetorum, adapt to increased drought
stress? Soil Biology & Biochemistry, 40(7), 1765-1771.
Marengo, J.A., J. Tomasella, L.M. Alves, W.R. Soares, and D.A. Rodriguez, 2011:
The drought of 2010 in the context of historical droughts in the Amazon
region. Geophysical Research Letters, 38(12), L12703, doi:10.1029/
2011GL047436.
Marini, L., M. Ayres, A. Battisti, and M. Faccoli, 2012: Climate affects severity and
altitudinal distribution of outbreaks in an eruptive bark beetle. Climatic Change,
115(2), 327-341.
Markovic, D., U. Scharfenberger, S. Schmutz, F. Pletterbauer, and C. Wolter, 2013:
Variability and alterations of water temperatures across the Elbe and Danube
River Basins. Climatic Change, 119(2), 375-389.
Marlon, J.R., P.J. Bartlein, M.K. Walsh, S.P. Harrison, K.J. Brown, M.E. Edwards, P.E.
Higuera, M.J. Power, R.S. Anderson, C. Briles, A. Brunelle, C. Carcaillet, M.
Daniels, F.S. Hu, M. Lavoie, C. Long, T. Minckley, P.J.H. Richard, A.C. Scott, D.S.
Shafer, W. Tinner, C.E. Umbanhowar, and C. Whitlock, 2009: Wildfire responses
to abrupt climate change in North America. Proceedings of the National
Academy of Sciences of the United States of America, 106(8), 2519-2524.
Marlon, J.R., P.J. Bartlein, A.-L. Daniau, S.P. Harrison, S.Y. Maezumi, M.J. Power, W.
Tinner, and B. Vanniére, 2013: Global biomass burning: a synthesis and review
of Holocene paleofire records and their controls. Quaternary Science Reviews,
65, 5-25.
Martí-Roura, M., P. Casals, and J. Romanyà, 2011: Temporal changes in soil organic
C under Mediterranean shrublands and grasslands: impact of fire and drought.
Plant and Soil, 338(1-2), 289-300.
Martin, D., T. Lal, C.B. Sachdev, and J.P. Sharma, 2010: Soil organic carbon storage
changes with climate change, landform and land use conditions in Garhwal hills
of the Indian Himalayan mountains. Agriculture, Ecosystems & Environment,
138(1-2), 64-73.
Martin, T.E. and J.L. Maron, 2012: Climate impacts on bird and plant communities
from altered animal-plant interactions. Nature Climate Change, 2(3), 195-200.
Martinez-Alier, J., 2011: The EROI of Agriculture and its use by the Via Campesina.
The Journal of Peasant Studies, 38(1), 145-160.
Martinez, P.J., 2012: Invasive crayfish in a high desert river: implications of concurrent
invaders and climate change. Aquatic Invasions, 7(2), 219-234.
Maseyk, K., D. Hemming, A. Angert, S.W. Leavitt, and D. Yakir, 2011: Increase in water-
use efficiency and underlying processes in pine forests across a precipitation
gradient in the dry Mediterranean region over the past 30 years. Oecologia,
167(2), 573-585.
Maslin, M., M. Owen, R. Betts, S. Day, T. Dunkley Jones, and A. Ridgwell, 2010: Gas
hydrates: past and future geohazard? Philosophical Transactions of the Royal
Society A, 368(1919), 2369-2393.
Mastrandrea, M.D., C.B. Field, T.F. Stocker, O. Edenhofer, K.L. Ebi, D.J. Frame, H. Held,
E. Kriegler, K.J. Mach, P.R. Matschoss, G.-K. Plattner, G.W. Yohe, and F.W. Zwiers,
2010: Guidance Note for Lead Authors of the IPCC Fifth Assessment Report on
Consistent Treatment of Uncertainties. Intergovernmental Panel on Climate
Change (IPCC), Geneva, Switzerland, 5 pp., www.ipcc.ch/pdf/supporting-
material/uncertainty-guidance-note.pdf.
Matthews, S.N., L.R. Iverson, A.M. Prasad, M.P. Peters, and P.G. Rodewald, 2011:
Modifying climate change habitat models using tree species-specific assessments
of model uncertainty and life history-factors. Forest Ecology and Management,
262(8), 1460-1472.
Matthiessen, B., E. Mielke, and U. Sommer, 2010: Dispersal decreases diversity in
heterogeneous metacommunities by enhancing regional competition. Ecology,
91(7), 2022-2033.
Mattila, N., V. Kaitala, A. Komonen, J. Paivinen, and J.S. Kotiaho, 2011: Ecological
correlates of distribution change and range shift in butterflies. Insect
Conservation and Diversity, 4(4), 239-246.
Matusick, G., K.X. Ruthrof, and G.S.J. Hardy, 2012: Drought and heat triggers sudden
and severe dieback in a dominant Mediterranean-type woodland species. Open
Journal of Forestry, 2(4), 183-186.
Matusick, G., K.X. Ruthrof, N.C. Brouwers, B. Dell, and G.S.J. Hardy, 2013: Sudden
forest canopy collapse corresponding with extreme drought and heat in a
Mediterranean-type eucalypt forest in southwestern Australia. European Journal
of Forest Research, 132(3), 497-510.
Matzek, V., 2012: Trait values, not trait plasticity, best explain invasive species’
performance in a changing environment. PLoS One, 7(10), e48821, doi:10.1371/
journal.pone.0048821.
Mayle, F.E. and M.J. Power, 2008: Impact of a drier Early-Mid-Holocene climate upon
Amazonian forests. Philosophical Transactions of the Royal Society B,
363(1498), 1829-1838.
McAlpine, C.A., J. Syktus, J.G. Ryan, R.C. Deo, G.M. Mckeon, H.A. Mcgowan, and S.R.
Phinn, 2009: A continent under stress: interactions, feedbacks and risks
associated with impact of modified land cover on Australia’s climate. Global
Change Biology, 15(9), 2206-2223.

347
Terrestrial and Inland Water Systems Chapter 4
4
McCain, C.M. and R.K. Colwell, 2011: Assessing the threat to montane biodiversity
from discordant shifts in temperature and precipitation in a changing climate.
Ecology Letters, 14(12), 1236-1245.
McCarthy, M.P., M.J. Best, and R.A. Betts, 2010: Climate change in cities due to
global warming and urban effects. Geophysical Research Letters, 37, L09705.
McDougall, K.L., J.M. Alexander, S. Haider, A. Pauchard, N.G. Walsh, and C. Kueffer,
2011: Alien flora of mountains: global comparisons for the development of
local preventive measures against plant invasions. Diversity and Distributions,
17(1), 103-111.
McDowell, N.G., D.J. Beerling, D.D. Breshears, R.A. Fisher, K.F. Raffa, and M. Stitt,
2011: The interdependence of mechanisms underlying climate-driven vegetation
mortality. Trends in Ecology & Evolution, 26(10), 523-532.
McGeoch, M.A., S.H.M. Butchart, D. Spear, E. Marais, E.J. Kleynhans, A. Symes, J.
Chanson, and M. Hoffmann, 2010: Global indicators of biological invasion:
species numbers, biodiversity impact and policy responses. Diversity and
Distributions, 16(1), 95-108.
McGuire, A.D., F.S. Chapin, C. Wirth, M. Apps, J. Bhatti, T. Callaghan, T.R. Christensen,
J.S. Clein, M. Fukuda, T. Maximov, A. Onuchin, A. Shvidenko, E. Vaganov, J.G.
Canadell, D.E. Pataki, and L.F. Pitelka, 2007: Responses of high latitude
ecosystems to global change: potential consequences for the climate system.
In: Terrestrial Ecosystems in a Changing World [Canadell, J.G., D.E. Pataki, and
L.F. Pitelka (eds.)]. Global Change – The IGBP Series, Springer-Verlag, Berlin,
Heidelberg, Germany, pp. 297-310.
McGuire, A.D., D.J. Hayes, D.W. Kicklighter, M. Manizza, Q. Zhuang, M. Chen, M.J.
Follows, K.R. Gurney, J.W. McClelland, J.M. Melillo, B.J. Peterson, and R.G. Prinn,
2010: An analysis of the carbon balance of the Arctic Basin from 1997 to 2006.
Tellus Series B:Chemical and Physical Meteorology, 62(5), 455-474.
McGuire, A.D., T.R. Christensen, D. Hayes, A. Heroult, E. Euskirchen, Y. Yi, J.S. Kimball,
C. Koven, P. Lafleur, P.A. Miller, W. Oechel, P. Peylin, and M. Williams, 2012:
An assessment of the carbon balance of arctic tundra: comparisons among
observations, process models, and atmospheric inversions. Biogeosciences
Discussions, 9(4), 4543-4594.
McKenzie, V.J. and A.C. Peterson, 2012: Pathogen pollution and the emergence of a
deadly amphibian pathogen. Molecular Ecology, 21(21), 5151-5154.
McKinney, M., 2008: Effects of urbanization on species richness: a review of plants
and animals. Urban Ecosystems, 11(2), 161-176.
McLachlan, J.S., J.S. Clark, and P.S. Manos, 2005: Molecular indicators of tree migration
capacity under rapid climate change. Ecology, 86(8), 2088-2098.
McLaughlin, S.B., M. Nosal, S.D. Wullschleger, and G. Sun, 2007: Interactive effects
of ozone and climate on tree growth and water use in a southern Appalachian
forest in the USA. New Phytologist, 174(1), 109-124.
McMahon, S.M., S.P. Harrison, W.S. Armbruster, P.J. Bartlein, C.M. Beale, M.E. Edwards,
J. Kattge, G. Midgley, X. Morin, and I.C. Prentice, 2011: Improving assessment
and modelling of climate change impacts on global terrestrial biodiversity.
Trends in Ecology & Evolution, 26(5), 249-259.
McMenamin, S.K. and L. Hannah, 2012: First extinctions on land. In: Saving a Million
Species: Extinction Risk from Climate Change [Hannah, L. (ed.)]. Island Press,
Washington, DC, USA, pp. 89-102.
Mehl, J.W., C.J. Geldenhuys, J. Roux, and M.J. Wingfield, 2010: Die-back of kiaat
(Pterocarpus angolensis) in southern Africa: a cause for concern? Southern
Forests: a Journal of Forest Science, 72(3-4), 121-132.
Meier, E.S., H. Lischke, D.R. Schmatz, and N.E. Zimmermann, 2012: Climate,
competition and connectivity affect future migration and ranges of European
trees. Global Ecology and Biogeography, 21(2), 164-178.
Menéndez, R., A. Gonzalez-Megias, O.T. Lewis, M.R. Shaw, and C.D. Thomas, 2008:
Escape from natural enemies during climate-driven range expansion: a case
study. Ecological Entomology, 33(3), 413-421.
Menge, D.N.L. and C.B. Field, 2007: Simulated global changes alter phosphorus
demand in annual grassland. Global Change Biology, 13(12), 2582-2591.
Menzel, A., T.H. Sparks, N. Estrella, E. Koch, A. Aasa, R. Ahas, K. Alm-Kubler, P. Bissolli,
O. Braslavska, A. Briede, F.M. Chmielewski, Z. Crepinsek, Y. Curnel, A. Dahl, C.
Defila, A. Donnelly, Y. Filella, K. Jatcza, F. Mage, A. Mestre, O. Nordli, J. Penuelas,
P. Pirinen, V. Remisova, H. Scheifinger, M. Striz, A. Susnik, A.J.H. Van Vliet, F.E.
Wielgolaski, S. Zach, and A. Zust, 2006: European phenological response to
climate change matches the warming pattern. Global Change Biology, 12(10),
1969-1976.
Mercado, L.M., N. Bellouin, S. Sitch, O. Boucher, C. Huntingford, M. Wild, and P.M.
Cox, 2009: Impact of changes in diffuse radiation on the global land carbon
sink. Nature, 458(7241), 1014-1017.
Merilä, J., 2012: Evolution in response to climate change: in pursuit of the missing
evidence. Bioessays, 34(9), 811-818.
Meyers, E.M., B. Dobrowski, and C.L. Tague, 2010: Climate change impacts on flood
frequency, intensity, and timing may affect trout species in Sagehen Creek,
California. Transactions of the American Fisheries Society, 139(6), 1657-
1664.
Meyfroidt, P. and E.F. Lambin, 2011: Global forest transition: prospects for an end
to deforestation. Annual Review of Environment and Resources, 36, 343-371.
Michaelian, M., E.H. Hogg, R.J. Hall, and E. Arsenault, 2011: Massive mortality of
aspen following severe drought along the southern edge of the Canadian
boreal forest. Global Change Biology, 17(6), 2084-2094.
Michalski, S.G., W. Durka, A. Jentsch, J. Kreyling, S. Pompe, O. Schweiger, E. Willner,
and C. Beierkuhnlein, 2010: Evidence for genetic differentiation and divergent
selection in an autotetraploid forage grass (Arrhenatherum elatius). TAG
Theoretical and Applied Genetics, 120(6), 1151-1162.
Michelutti, N., A.P. Wolfe, R.D. Vinebrooke, B. Rivard, and J.P. Briner, 2005: Recent
primary production increases in arctic lakes. Geophysical Research Letters,
32(19), L19715, doi:10.1029/2005GL023693.
Midgley, G.F., I.D. Davies, C.H. Albert, R. Altwegg, L. Hannah, G.O. Hughes, L.R.
O’Halloran, C. Seo, J.H. Thorne, and W. Thuiller, 2010: BioMove – an integrated
platform simulating the dynamic response of species to environmental change.
Ecography, 33(3), 612-616.
Mihoub, J.B., N.G. Mouawad, P. Pilard, F. Jiguet, M. Low, and C. Teplitsky, 2012: Impact
of temperature on the breeding performance and selection patterns in lesser
kestrels Falco naumanni. Journal of Avian Biology, 43(5), 472-480.
Miles, L., A.C. Newton, R.S. DeFries, C. Ravilious, I. May, S. Blyth, V. Kapos, and J.E.
Gordon, 2006: A global overview of the conservation status of tropical dry
forests. Journal of Biogeography, 33(3), 491-505.
Millar, C., R. Westfall, D. Delany, J. King, and L. Graumlich, 2004: Response of
subalpine conifers in the Sierra Nevada, California, U.S.A., to 20
th
-century warming
and decadal climate variability. Arctic, Antarctic, and Alpine Research, 36(2),
181-200.
Millar, C.I., R.D. Westfall, D.L. Delany, M.J. Bokach, A.L. Flint, and L.E. Flint, 2012:
Forest mortality in high-elevation whitebark pine (Pinus albicaulis) forests of
eastern California, USA; influence of environmental context, bark beetles,
climatic water deficit, and warming. Canadian Journal of Forest Research /
Revue Canadienne De Recherche Forestiere, 42(4), 749-765.
Millennium Ecosystem Assessment, 2003: Ecosystems and Human Well-being: A
Framework for Assessment. Island Press, Washington, DC, USA, 212 pp.
Millennium Ecosystem Assessment, 2005a: Ecosystems and Human Well-being:
Desertification Synthesis. World Resources Institute, Washington, DC, USA,
26 pp.
Millennium Ecosystem Assessment, 2005b: Ecosystems and Human Well-being:
Biodiversity Synthesis. World Resources Institute, Washington, DC, USA, 86 pp.
Millennium Ecosystem Assessment, 2005c: Ecosystems and Human Well-being:
Current State and Trends, Vol. 1. Island Press, Washington, DC, USA, 917 pp.
Millennium Ecosystem Assessment, 2005d: Ecosystems and Human Well-being:
Scenerios, Vol. 2. Island Press, Washington, DC, USA, 560 pp.
Millennium Ecosystem Assessment, 2005e: Ecosystems and Human Well-being:
Policy Responses, Vol. 3. Island Press, Washington, DC, USA, 621 pp.
Miller-Rushing, A.J., T.L. Lloyd-Evans, R.B. Primack, and P. Satzinger, 2008: Bird
migration times, climate change, and changing population sizes. Global Change
Biology, 14(9), 1959-1972.
Mills, G., F. Hayes, S. Wilkinson, and W.J. Davies, 2009: Chronic exposure to increasing
background ozone impairs stomatal functioning in grassland species. Global
Change Biology, 15(6), 1522-1533.
Mills, G., F. Hayes, D. Simpson, L. Emberson, D. Norris, H. Harmens, and P. Buker, 2011:
Evidence of widespread effects of ozone on crops and (semi-)natural vegetation
in Europe (1990-2006) in relation to AOT40-and flux-based risk maps. Global
Change Biology, 17(1), 592-613.
Minnich, R.A., 2007: Southern California conifer forests. In: Terrestrial Vegetation of
California [Barbour, M., T. Keeler-Wolf, and A. A. Schoenherr (eds.)]. University
of California Press, Berkeley, CA, USA, pp. 502-538.
Minteer, B.A. and J.P. Collins, 2010: Move it or lose it? The ecological ethics of
relocating species under climate change. Ecological Applications, 20(7), 1801-
1804.
Miranda, J.D., F.M. Padilla, and F.I. Pugnaire, 2009: Response of a Mediterranean
semiarid community to changing patterns of water supply. Perspectives in Plant
Ecology Evolution and Systematics, 11(4), 255-266.

348
Chapter 4 Terrestrial and Inland Water Systems
4
Mishra, V., K.A. Cherkauer, D. Niyogi, M. Lei, B.C. Pijanowski, D.K. Ray, L.C. Bowling,
and G. Yang, 2010: A regional scale assessment of land use/land cover and
climatic changes on water and energy cycle in the upper Midwest United States.
International Journal of Climatology, 30(13), 2025-2044.
Mitas, C.M. and A. Clement, 2005: Has the Hadley cell been strengthening in recent
decades? Geophysical Research Letters, 32(3), L030809, doi:10.1029/
2004GL021765.
Mitchard, E.T.A., S.S. Saatchi, F.F. Gerard, S.L. Lewis, and P. Meir, 2009: Measuring
woody encroachment along a forest-savanna boundary in Central Africa. Earth
Interactions, 13(8), 1-29.
Mitchell, T.D. and P.D. Jones, 2005: An improved method of constructing a database of
monthly climate observations and associated high-resolution grids. International
Journal of Climatology, 25(6), 693-712.
Miyake, S., M. Renouf, A. Peterson, C. McAlpine, and C. Smith, 2012: Land-use and
environmental pressures resulting from current and future bioenergy crop
expansion: a review. Journal of Rural Studies, 28(4), 650-658.
Mohan, J.E., L.H. Ziska, W.H. Schlesinger, R.B. Thomas, R.C. Sicher, K. George, and J.S.
Clark, 2006: Biomass and toxicity responses of poison ivy (Toxicodendron
radicans) to elevated atmospheric CO
2
. Proceedings of the National Academy
of Sciences of the United States of America, 103(24), 9086-9089.
Moiseyev, A., B. Solberg, A.M.I. Kallio, and M. Lindner, 2011: An economic analysis
of the potential contribution of forest biomass to the EU RES target and its
implications for the EU forest industries. Journal of Forest Economics, 17(2),
197-213.
Moleele, N.M., S. Ringrose, W. Matheson, and C. Vanderpost, 2002: More woody
plants? The status of bush encroachment in Botswana’s grazing areas. Journal
of Environmental Management, 64(1), 3-11.
Møller, A.P., D. Rubolini, and E. Lehikoinen, 2008: Populations of migratory bird
species that did not show a phenological response to climate change are
declining. Proceedings of the National Academy of Sciences of the United States
of America, 105(42), 16195-16200.
Monahan, W.B. and M.W. Tingley, 2012: Niche tracking and rapid establishment of
distributional equilibrium in the house sparrow show potential responsiveness
of species to climate change. PLoS One, 7(7), e42097, doi:10.1371/
journal.pone.0042097.
Monteith, D.T., J.L. Stoddard, C.D. Evans, H.A. de Wit, M. Forsius, T. Hogasen, A.
Wilander, B.L. Skjelkvale, D.S. Jeffries, J. Vuorenmaa, B. Keller, J. Kopacek, and J.
Vesely, 2007: Dissolved organic carbon trends resulting from changes in
atmospheric deposition chemistry. Nature, 450(7169), 537-540.
Montoya, J.M. and D. Raffaelli, 2010: Climate change, biotic interactions and
ecosystem services. Philosophical Transactions of the Royal Society B,
365(1549), 2013-2018.
Mooney, H.A., S.H. Bullock, and E. Medina, 1995: Introduction. In: Seasonally Dry
Tropical Forests [Bullock, S.H., H.A. Mooney, and E. Medina (eds.)]. Cambridge
University Press, Cambridge, UK, pp. 1-8.
Moore, S., C.D. Evans, S.E. Page, M.H. Garnett, T.G. Jones, C. Freeman, A. Hooijer, A.J.
Wiltshire, S.H. Limin, and V. Gauci, 2013: Deep instability of deforested tropical
peatlands revealed by fluvial organic carbon fluxes. Nature, 493(7434), 660-663.
Morecroft, M.D., H.Q.P. Crick, S.J. Duffield, and N.A. Macgregor, 2012: Resilience to
climate change: translating principles into practice. Journal of Applied Ecology,
49(3), 547-551.
Morin, X. and W. Thuiller, 2009: Comparing niche- and process-based models to
reduce prediction uncertainty in species range shifts under climate change.
Ecology, 90(5), 1301-1313.
Morin, X., C. Augspurger, and I. Chuine, 2007: Process-based modeling of species’
distributions: what limits temperate tree species’ range boundaries? Ecology,
88(9), 2280-2291.
Moritz, C. and R. Agudo, 2013: The future of species under climate change: resilience
or decline? Science, 341(6145), 504-508.
Moritz, M.A., M.A. Parisien, E. Batllori, M.A. Krawchuk, J. Van Dorn, D.J. Ganz, and K.
Hayhoe, 2012: Climate change and disruptions to global fire activity. Ecosphere,
3(6), 49, doi:10.1890/ES11-00345.
Morrison, J., M.C. Quick, and M.G.G. Foreman, 2002: Climate change in the Fraser
River watershed: flow and temperature projections. Journal of Hydrology,
263(1-4), 230-244.
Mueller, A.D., G.A. Islebe, M.B. Hillesheim, D.A. Grzesik, F.S. Anselmetti, D. Ariztegui,
M. Brenner, J.H. Curtis, D.A. Hodell, and K.A. Venz, 2009: Climate drying and
associated forest decline in the lowlands of northern Guatemala during the
late Holocene. Quaternary Research, 71(2), 133-141.
Mueller, D.R., P. Van Hove, D. Antoniades, M.O. Jeffries, and W.F. Vincent, 2009: High
Arctic lakes as sentinel ecosystems: cascading regime shifts in climate, ice cover,
and mixing. Limnology and Oceanography, 54(6), 2371-2385.
Mueller, R.C., C.M. Scudder, M.E. Porter, R.T. Trotter, C.A. Gehring, and T.G. Whitham,
2005: Differential tree mortality in response to severe drought: evidence for
long-term vegetation shifts. Journal of Ecology, 93(6), 1085-1093.
Muhlfeld, C.C., J.J. Giersch, F.R. Hauer, G.T. Pederson, G. Luikart, D.P. Peterson, C.C.
Downs, and D.B. Fagre, 2011: Climate change links fate of glaciers and an
endemic alpine invertebrate. Climatic Change, 106(2), 337-345.
Muñoz-Robles, C., N. Reid, M. Tighe, S.V. Briggs, and B. Wilson, 2011: Soil hydrological
and erosional responses in areas of woody encroachment, pasture and
woodland in semi-arid Australia. Journal of Arid Environments, 75(10), 936-945.
Murdiyarso, D., K. Hergoualc’h, and L.V. Verchot, 2010: Opportunities for reducing
greenhouse gas emissions in tropical peatlands. Proceedings of the National
Academy of Sciences of the United States of America, 107(46), 19655-19660.
Musolin, D.L., D. Tougou, and K. Fujisaki, 2010: Too hot to handle? Phenological and
life-history responses to simulated climate change of the southern green stink
bug Nezara viridula (Heteroptera: Pentatomidae). Global Change Biology,
16(1), 73-87.
Myers-Smith, I.H., B.C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K.D. Tape,
M. Macias-Fauria, U. Sass-Klaassen, E. Levesque, S. Boudreau, P. Ropars, L.
Hermanutz, A. Trant, L.S. Collier, S. Weijers, J. Rozema, S.A. Rayback, N.M.
Schmidt, G. Schaepman-Strub, S. Wipf, C. Rixen, C.B. Menard, S. Venn, S. Goetz,
L. Andreu-Hayles, S. Elmendorf, V. Ravolainen, J. Welker, P. Grogan, H.E. Epstein,
and D.S. Hik, 2011: Shrub expansion in tundra ecosystems: dynamics, impacts
and research priorities. Environmental Research Letters, 6(4), 045509,
doi:10.1088/1748-9326/6/4/045509.
Nabuurs, G.J., G.M. Hengeveld, D.C. van der Werf, and A.H. Heidema, 2010: European
forest carbon balance assessed with inventory based methods – an introduction
to a special section. Forest Ecology and Management, 260(3), 239-240.
Naito, A.T. and D.M. Cairns, 2011: Patterns and processes of global shrub expansion.
Progress in Physical Geography, 35(4), 423-442.
Nakazawa, T. and H. Doi, 2012: A perspective on match/mismatch of phenology in
community contexts. Oikos, 121(4), 489-495.
Nathan, R., 2006: Long-distance dispersal of plants. Science, 313(5788), 786-788.
Nathan, R., N. Horvitz, Y.P. He, A. Kuparinen, F.M. Schurr, and G.G. Katul, 2011: Spread
of North American wind-dispersed trees in future environments. Ecology Letters,
14(3), 211-219.
Nemet, G.F., 2009: Net radiative forcing from widespread deployment of
photovoltaics. Environmental Science and Technology, 43(6), 2173-2178.
Nepstad, D.C., I.M. Tohver, D. Ray, P. Moutinho, and G. Cardinot, 2007: Mortality of
large trees and lianas following experimental drought in an Amazon forest.
Ecology, 88(9), 2259-2269.
Nepstad, D.C., C.M. Stickler, B. Soares, and F. Merry, 2008: Interactions among
Amazon land use, forests and climate: prospects for a near-term forest tipping
point. Philosophical Transactions of the Royal Society B, 363(1498), 1737-1746.
Nepstad, D.C., B.S. Soares, F. Merry, A. Lima, P. Moutinho, J. Carter, M. Bowman, A.
Cattaneo, H. Rodrigues, S. Schwartzman, D.G. McGrath, C.M. Stickler, R.
Lubowski, P. Piris-Cabezas, S. Rivero, A. Alencar, O. Almeida, and O. Stella, 2009:
The end of deforestation in the Brazilian Amazon. Science, 326(5958), 1350-
1351.
Nepstad, D.C., W. Boyd, C.M. Stickler, T. Bezerra, and A.A. Azevedo, 2013: Responding
to climate change and the global land crisis: REDD+, market transformation
and low-emissions rural development. Philosophical Transactions of the Royal
Society B, 368(1619), 20120167, doi:10.1098/rstb.2012.0167.
Ni, J., 2011: Impacts of climate change on Chinese ecosystems: key vulnerable regions
and potential thresholds. Regional Environmental Change, 11, S49-S64.
Niboyet, A., J.R. Brown, P. Dijkstra, J.C. Blankinship, P.W. Leadley, X. Le Roux, L.
Barthes, R.L. Barnard, C.B. Field, and B.A. Hungate, 2011: Global change could
amplify fire effects on soil greenhouse gas emissions. PLoS One, 6(6), e20105,
doi:10.1371/journal.pone.0020105.
Nicholls, R.J., 2004: Coastal flooding and wetland loss in the 21
st
century: changes
under the SRES climate and socio-economic scenarios. Global Environmental
Change: Human and Policy Dimensions, 14(1), 69-86.
Nielsen, U.N. and D.H. Wall, 2013: The future of soil invertebrate communities in
polar regions: different climate change responses in the Arctic and Antarctic?
Ecology Letters, 16(3), 409-419.
Nilsson, C., C.A. Reidy, M. Dynesius, and C. Revenga, 2005: Fragmentation and flow
regulation of the world’s large river systems. Science, 308(5720), 405-408.

349
Terrestrial and Inland Water Systems Chapter 4
4
Nock, C.A., P.J. Baker, W. Wanek, A. Leis, M. Grabner, S. Bunyavejchewin, and P. Hietz,
2011: Long-term increases in intrinsic water-use efficiency do not lead to
increased stem growth in a tropical monsoon forest in western Thailand. Global
Change Biology, 17(2), 1049-1063.
Nogues-Bravo, D., R. Ohlemuller, P. Batra, and M.B. Araujo, 2010: Climate predictors
of Late Quaternary extinctions. Evolution, 64(8), 2442-2449.
Norberg, J., M.C. Urban, M. Vellend, C.A. Klausmeier, and N. Loeuille, 2012: Eco-
evolutionary responses of biodiversity to climate change. Nature Climate
Change, 2(10), 747-751.
Norby, R.J. and D.R. Zak, 2011: Ecological lessons from free-air CO
2
enrichment
(FACE) experiments. Annual Review of Ecology, Evolution, and Systematics,
42, 181-203.
Normand, S., U.A. Treier, C. Randin, P. Vittoz, A. Guisan, and J.C. Svenning, 2009:
Importance of abiotic stress as a range-limit determinant for European plants:
insights from species responses to climatic gradients. Global Ecology and
Biogeography, 18(4), 437-449.
Nowicki, P., A. Pepkowska, J. Kudlek, P. Skorka, M. Witek, J. Settele, and M.
Woyciechowski, 2007: From metapopulation theory to conservation
recommendations: lessons from spatial occurrence and abundance patterns of
Maculinea butterflies. Biological Conservation, 140(1-2), 119-129.
O’Connor, F.M., O. Boucher, N. Gedney, C.D. Jones, G.A. Folberth, R. Coppell, P.
Friedlingstein, W.J. Collins, J. Chappellaz, J. Ridley, and C.E. Johnson, 2010:
Possible role of wetlands, permafrost, and methane hydrates in the methane
cycle under future climate change: a review. Reviews of Geophysics, 48(4),
RG4005, doi:10.1029/2010RG000326.
O’Donnell, J.A., J.W. Harden, A.D. McGuire, M.Z. Kanevskiy, M.T. Jorgenson, and X.M.
Xu, 2011: The effect of fire and permafrost interactions on soil carbon
accumulation in an upland black spruce ecosystem of interior Alaska: implications
for post-thaw carbon loss. Global Change Biology, 17(3), 1461-1474.
O’Halloran, T.L., B.E. Law, M.L. Goulden, Z. Wang, J.G. Barr, C. Schaaf, M. Brown, J.D.
Fuentes, M. Göckede, A. Black, and V. Engel, 2012: Radiative forcing of natural
forest disturbances. Global Change Biology, 18(2), 555-565.
O’Reilly, C.M., S.R. Alin, P.D. Plisnier, A.S. Cohen, and B.A. McKee, 2003: Climate
change decreases aquatic ecosystem productivity of Lake Tanganyika, Africa.
Nature, 424(6950), 766-768.
Obersteiner, M., H. Böttcher, and Y. Yamagata, 2010: Terrestrial ecosystem management
for climate change mitigation. Current Opinion in Environmental Sustainability,
2(4), 271-276.
OECD and FAO, 2010: OECD-FAO Agricultural Outlook 2010-2019. Organisation for
Economic Co-operation and Development (OECD) and the Food and Agriculture
Organization of the United Nations (FAO), OECD Publishing, Paris, France, 247 pp.
Ogawa-Onishi, Y., P.M. Berry, and N. Tanaka, 2010: Assessing the potential impacts
of climate change and their conservation implications in Japan: a case study
of conifers. Biological Conservation, 143(7), 1728-1736.
Oleszczuk, R., K. Regina, L. Szajdak, H. Höper, and V. Maryganova, 2008: Impacts of
agricultural utilization of peat soils on the greenhouse gas balance. In: Peatlands
and Climate Change [Strack, M. (ed.)]. International Peat Society, Jyväskylä,
Finland, pp. 70-97.
Oliver, R.J., J.W. Finch, and G. Taylor, 2009: Second generation bioenergy crops and
climate change: a review of the effects of elevated atmospheric CO
2
and
drought on water use and the implications for yield. Global Change Biology
Bioenergy, 1(2), 97-114.
Oliver, T., J.K. Hill, C.D. Thomas, T. Brereton, and D.B. Roy, 2009: Changes in habitat
specificity of species at their climatic range boundaries. Ecology Letters, 12(10),
1091-1102.
Oliver, T.H., S. Gillings, M. Girardello, G. Rapacciuolo, T.M. Brereton, G.M. Siriwardena,
D.B. Roy, R. Pywell, and R.J. Fuller, 2012a: Population density but not stability
can be predicted from species distribution models. Journal of Applied Ecology,
49(3), 581-590.
Oliver, T.H., R.J. Smithers, S. Bailey, C.A. Walmsley, and K. Watts, 2012b: A decision
framework for considering climate change adaptation in biodiversity
conservation planning. Journal of Applied Ecology, 49(6), 1247-1255.
Oltmans, S.J., A.S. Lefohn, J.M. Harris, D.W. Tarasick, A.M. Thompson, H. Wernli, B.J.
Johnson, P.C. Novelli, S.A. Montzka, J.D. Ray, L.C. Patrick, C. Sweeney, A. Jefferson,
T. Dann, and J. Davies, 2006: Long term changes in tropospheric ozone.
Atmospheric Environment, 40(17), 3156-3173.
Ooi, M.K.J., T.D. Auld, and A.J. Denham, 2009: Climate change and bet-hedging:
interactions between increased soil temperatures and seed bank persistence.
Global Change Biology, 15(10), 2375-2386.
Ormerod, S.J., 2009: Climate change, river conservation and the adaptation challenge.
Aquatic Conservation: Marine and Freshwater Ecosystems, 19(6), 609-613.
Osawa, A., O.A. Zyryanova, Y. Matsuura, T. Kajimoto, and R.W. Wein, 2010: Permafrost
Ecosystems: Siberian Larch Forests. Springer, New York, NY, USA, 502 pp.
Ozgul, A., D.Z. Childs, M.K. Oli, K.B. Armitage, D.T. Blumstein, L.E. Olson, S. Tuljapurkar,
and T. Coulson, 2010: Coupled dynamics of body mass and population growth
in response to environmental change. Nature, 466(7305), 482-485.
Pacifici, M., L. Santini, M. Di Marco, D. Baisero, L. Francucci, G. Grottolo Marassini, P.
Visconti, and C. Rondinini, 2013: Generation lengths for mammals. Nature
Conservation, 5(2013), 89-94, doi:10.3897/natureconservation.5.5734.
Paerl, H.W., N.S. Hall, and E.S. Calandrino, 2011: Controlling harmful cyanobacterial
blooms in a world experiencing anthropogenic and climatic-induced change.
Science of the Total Environment, 409(10), 1739-1745.
Page, S.E., F. Siegert, J.O. Rieley, H.D. Boehm, A. Jaya, and S. Limin, 2002: The amount
of carbon released from peat and forest fires in Indonesia during 1997. Nature,
420(6911), 61-65.
Page, S.E., J.O. Rieley, and C.J. Banks, 2011: Global and regional importance of the
tropical peatland carbon pool. Global Change Biology, 17(2), 798-818.
Pan, Y., R. Birdsey, J. Hom, and K. McCullough, 2009: Separating effects of changes
in atmospheric composition, climate and land-use on carbon sequestration of
US Mid-Atlantic temperate forests. Forest Ecology and Management, 259(2),
151-164.
Pan, Y., R. Birdsey, J. Fang, R. Houghton, P. Kauppi, W.A. Kurz, O.L. Phillips, A. Shvidenko,
S.L. Lewis, J.G. Canadell, P. Ciais, R.B. Jackson, S. Pacala, A.D. McGuire, S. Piao,
A. Rautiainen, S. Sitch, and D. Hayes, 2011: A large and persistent carbon sink
in the world’s forests. Science, 333(6045), 988-993.
Parent, M.B. and D. Verbyla, 2010: The browning of Alaska’s boreal forest. Remote
Sensing, 2(12), 2729-2747.
Parker-Allie, F., C.F. Musil, and W. Thuiller, 2009: Effects of climate warming on the
distributions of invasive Eurasian annual grasses: a South African perspective.
Climatic Change, 94(1-2), 87-103.
Parker, B.R., R.D. Vinebrooke, and D.W. Schindler, 2008: Recent climate extremes alter
alpine lake ecosystems. Proceedings of the National Academy of Sciences of
the United States of America, 105(35), 12927-12931.
Parmesan, C., 2006: Ecological and evolutionary responses to recent climate change.
Annual Review of Ecology Evolution and Systematics, 37, 637-669.
Parmesan, C., 2007: Influences of species, latitudes and methodologies on estimates
of phenological response to global warming. Global Change Biology, 13(9),
1860-1872.
Parmesan, C. and G. Yohe, 2003: A globally coherent fingerprint of climate change
impacts across natural systems. Nature, 421(6918), 37-42.
Pataki, D.E., M.M. Carreiro, J. Cherrier, N.E. Grulke, V. Jennings, S. Pincetl, R.V. Pouyat,
T.H. Whitlow, and W.C. Zipperer, 2011: Coupling biogeochemical cycles in urban
environments: ecosystem services, green solutions, and misconceptions.
Frontiers in Ecology and the Environment, 9(1), 27-36.
Pateman, R.M., J.K. Hill, D.B. Roy, R. Fox, and C.D. Thomas, 2012: Temperature-
dependent alterations in host use drive rapid range expansion in a butterfly.
Science, 336(6084), 1028-1030.
Paterson, J.S., M.B. Araujo, P.M. Berry, J.M. Piper, and M.D.A. Rounsevell, 2008:
Mitigation, adaptation, and the threat to biodiversity. Conservation Biology,
22(5), 1352-1355.
Pauli, H., M. Gottfried, K. Reiter, C. Klettner, and G. Grabherr, 2007: Signals of range
expansions and contractions of vascular plants in the high Alps: observations
(1994-2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Global
Change Biology, 13, 147-156.
Pauli, H., M. Gottfried, S. Dullinger, O. Abdaladze, M. Akhalkatsi, J.L.B. Alonso, G. Coldea,
J. Dick, B. Erschbamer, R.F. Calzado, D. Ghosn, J.I. Holten, R. Kanka, G. Kazakis, J.
Kollar, P. Larsson, P. Moiseev, D. Moiseev, U. Molau, J.M. Mesa, L. Nagy, G. Pelino,
M. Puscas, G. Rossi, A. Stanisci, A.O. Syverhuset, J.P. Theurillat, M. Tomaselli, P.
Unterluggauer, L. Villar, P. Vittoz, and G. Grabherr, 2012: Recent plant diversity
changes on Europe’s mountain summits. Science, 336(6079), 353-355.
Pauls, S.U., C. Nowak, M. Bálint, and M. Pfenninger, 2013: The impact of global climate
change on genetic diversity within populations and species. Molecular Ecology,
22(4), 925-946.
Paun, O., R.M. Bateman, M.F. Fay, M. Hedren, L. Civeyrel, and M.W. Chase, 2010: Stable
epigenetic effects impact adaptation in allopolyploid Orchids (Dactylorhiza:
Orchidaceae). Molecular Biology and Evolution, 27(11), 2465-2473.
Payette, S., 2007: Contrasted dynamics of northern Labrador tree lines caused by
climate change and migrational lag. Ecology, 88(3), 770-780.

350
Chapter 4 Terrestrial and Inland Water Systems
4
Payette, S. and L. Filion, 1985: White spruce expansion at the tree line and recent
climatic change. Canadian Journal of Forest Research / Revue Canadienne De
Recherche Forestiere, 15(1), 241-251.
Pearce-Higgins, J.W., L. Stephen, A. Douse, and R.H.W. Langston, 2012: Greater
impacts of wind farms on bird populations during construction than subsequent
operation: results of a multi-site and multi-species analysis. Journal of Applied
Ecology, 49(2), 386-394.
Pearlstine, L.G., E.V. Pearlstine, and N.G. Aumen, 2010: A review of the ecological
consequences and management implications of climate change for the Everglades.
Journal of the North American Benthological Society, 29(4), 1510-1526.
Pearman, P.B., C.F. Randin, O. Broennimann, P. Vittoz, W.O. van der Knaap, R. Engler,
G. Le Lay, N.E. Zimmermann, and A. Guisan, 2008: Prediction of plant species
distributions across six millennia. Ecology Letters, 11(4), 357-369.
Pearson, R.G., 2006: Climate change and the migration capacity of species. Trends
in Ecology & Evolution, 21(3), 111-113.
Pearson, R.G., 2011: Driven to Extinction: The Impact of Climate Change on
Biodiversity. Sterling, New York, NY, USA, 263 pp.
Pearson, R.G. and T.P. Dawson, 2003: Predicting the impacts of climate change on
the distribution of species: are bioclimate envelope models useful? Global
Ecology and Biogeography, 12(5), 361-371.
Pearson, R.G., S.J. Phillips, M.M. Loranty, P.S.A. Beck, T. Damoulas, S.J. Knight, and
S.J. Goetz, 2013: Shifts in Arctic vegetation and associated feedbacks under
climate change. Nature Climate Change, 3, 673-677.
Pechony, O. and D.T. Shindell, 2010: Driving forces of global wildfires over the past
millennium and the forthcoming century. Proceedings of the National Academy
of Sciences of the United States of America, 107(45), 19167-19170.
Pederson, G.T., L.J. Graumlich, D.B. Fagre, T. Kipfer, and C.C. Muhlfeld, 2010: A century
of climate and ecosystem change in Western Montana: what do temperature
trends portend? Climatic Change, 98(1-2), 133-154.
Pedlar, J.H., D.W. McKenney, I. Aubin, T. Beardmore, J. Beaulieu, L. Iverson, G.A.
O’Neill, R.S. Winder, and C. Ste-Marie, 2012: Placing forestry in the assisted
migration debate. BioScience, 62(9), 835-842.
Peltzer, D.A., R.B. Allen, G.M. Lovett, D. Whitehead, and D.A. Wardle, 2010: Effects of
biological invasions on forest carbon sequestration. Global Change Biology,
16(2), 732-746.
Peng, C., X. Zhou, S. Zhao, X. Wang, B. Zhu, S. Piao, and J. Fang, 2009: Quantifying
the response of forest carbon balance to future climate change in NE China:
model validation and prediction. Global and Planetary Change, 66(3-4), 179-194.
Peng, C., Z. Ma, X. Lei, Q. Zhu, H. Chen, W. Wang, S. Liu, W. Li, X. Fang, and X. Zhou,
2011: A drought-induced pervasive increase in tree mortality across Canada’s
boreal forests. Nature Climate Change, 1(9), 467-471.
Peng, J., W. Dong, Y. Wenping, Y. Zhang, and J. Li, 2013: Effects of increased CO
2
on
land water balance from 1850 to 1989. Theoretical and Applied Climatology,
111(3-4), 483-495.
Peñuelas, J. and M. Boada, 2003: A global change-induced biome shift in the
Montseny mountains (NE Spain). Global Change Biology, 9(2), 131-140.
Peñuelas, J., C. Gordon, L. Llorens, T. Nielsen, A. Tietema, C. Beier, P. Bruna, B. Emmett,
M. Estiarte, and A. Gorissen, 2004: Nonintrusive field experiments show different
plant responses to warming and drought among sites, seasons, and species in
a north-south European gradient. Ecosystems, 7(6), 598-612.
Peñuelas, J., P. Prieto, C. Beier, C. Cesaraccio, P. de Angelis, G. de Dato, B.A. Emmett,
M. Estiarte, J. Garadnai, A. Gorissen, E.K. Lang, G. Kroel-Dulay, L. Llorens, G.
Pellizzaro, T. Riis-Nielsen, I.K. Schmidt, C. Sirca, A. Sowerby, D. Spano, and A.
Tietema, 2007: Response of plant species richness and primary productivity in
shrublands along a north-south gradient in Europe to seven years of
experimental warming and drought: reductions in primary productivity in the
heat and drought year of 2003. Global Change Biology, 13(12), 2563-2581.
Peñuelas, J., J.G. Canadell, and R. Ogaya, 2011: Increased water-use efficiency during
the 20
th
century did not translate into enhanced tree growth. Global Ecology
and Biogeography, 20(4), 597-608.
Peñuelas, J., J. Sardans, A. Rivas-Ubach, and I.A. Janssens, 2012: The human-induced
imbalance between C, N and P in Earth’s life system. Global Change Biology,
18(1), 3-6.
Peñuelas, J., J. Sardans, M. Estiarte, R. Ogaya, J. Carnicer, M. Coll, A. Barbeta, A. Rivas-
Ubach, J. Llusia, M. Garbulsky, I. Filella, and A.S. Jump, 2013: Evidence of current
impact of climate change on life: a walk from genes to the biosphere. Global
Change Biology, 19(8), 2303-2338.
Pereira, H.M., P.W. Leadley, V. Proenca, R. Alkemade, J.P.W. Scharlemann, J.F. Fernandez-
Manjarres, M.B. Araujo, P. Balvanera, R. Biggs, W.W.L. Cheung, L. Chini, H.D.
Cooper, E.L. Gilman, S. Guenette, G.C. Hurtt, H.P. Huntington, G.M. Mace, T.
Oberdorff, C. Revenga, P. Rodrigues, R.J. Scholes, U.R. Sumaila, and M. Walpole,
2010: Scenarios for global biodiversity in the 21
st
century. Science, 330(6010),
1496-1501.
Péron, G., J.E. Hines, J.D. Nichols, W.L. Kendall, K.A. Peters, and D.S. Mizrahi, 2013:
Estimation of bird and bat mortality at wind-power farms with superpopulation
models. Journal of Applied Ecology, 50(4), 902-911.
Perry, L.G., D.C. Andersen, L.V. Reynolds, S.M. Nelson, and P.B. Shafroth, 2012:
Vulnerability of riparian ecosystems to elevated CO
2
and climate change in
arid and semiarid western North America. Global Change Biology, 18(3), 821-
842.
Peterken, G.F. and E.P. Mountford, 1996: Effects of drought on beech in Lady Park
Wood, an unmanaged mixed deciduous woodland. Forestry, 69(2), 125-136.
Peters, D.P.C., J.E. Herrick, H.C. Monger, and H.T. Huang, 2010: Soil-vegetation-climate
interactions in arid landscapes: effects of the North American monsoon on grass
recruitment. Journal of Arid Environments, 74(5), 618-623.
Peterson, M.A. and R.F. Denno, 1998: The influence of dispersal and diet breadth on
patterns of genetic isolation by distance in phytophagous insects. American
Naturalist, 152(3), 428-446.
Peterson, A.T., A. Stewart, K.I. Mohamed, and M.B. Araujo, 2008: Shifting global
invasive potential of European plants with climate change. PLoS One, 3(6),
e2441, doi:10.1371/journal.pone.0002441.
Peterson, A.T.S., J., R.G. Pearson, R.P. Anderson, E. Martínez-Meyer, M. Nakamura,
and M.B. Araújo, 2011: Ecological Niches and Geographic Distributions. Princeton
University Press, Princeton, NJ, USA, 328 pp.
Petit, B. and F. Montagnini, 2006: Growth in pure and mixed plantations of tree
species used in reforesting rural areas of the humid region of Costa Rica,
Central America. Forest Ecology and Management, 233(2-3), 338-343.
Petitpierre, B., C. Kueffer, O. Broennimann, C. Randin, C. Daehler, and A. Guisan,
2012: Climatic niche shifts are rare among terrestrial plant invaders. Science,
335(6074), 1344-1348.
Peylin, P., P. Bousquet, C. Le Quere, P. Friedlingstein, G. McKinley, N. Gruber, P. Rayner,
and P. Ciais, 2005: Multiple constraints on regional CO
2
flux variations over
land and oceans. Global Biogeochemical Cycles, 19(1), GB1011, doi:10.1029/
2003GB002214.
Phillimore, A.B., J.D. Hadfield, O.R. Jones, and R.J. Smithers, 2010: Differences in
spawning date between populations of common frog reveal local adaptation.
Proceedings of the National Academy of Sciences of the United States of
America, 107(18), 8292-8297.
Phillips, O.L., R.V. Martinez, L. Arroyo, T.R. Baker, T. Killeen, S.L. Lewis, Y. Malhi, A.M.
Mendoza, D. Neill, P.N. Vargas, M. Alexiades, C. Ceron, A. Di Fiore, T. Erwin, A.
Jardim, W. Palacios, M. Saldias, and B. Vinceti, 2002: Increasing dominance of
large lianas in Amazonian forests. Nature, 418(6899), 770-774.
Phillips, O.L., R. Vásquez Martínez, A. Monteagudo Mendoza, T. Baker, and P. Núñez-
Vargas, 2005: Large lianas as hyperdynamic elements of the tropical forest
canopy. Ecology, 86(5), 1250-1258.
Phillips, O.L., L. Aragao, S.L. Lewis, J.B. Fisher, J. Lloyd, G. Lopez-Gonzalez, Y. Malhi, A.
Monteagudo, J. Peacock, C.A. Quesada, G. van der Heijden, S. Almeida, I. Amaral,
L. Arroyo, G. Aymard, T.R. Baker, O. Banki, L. Blanc, D. Bonal, P. Brando, J. Chave,
A.C.A. de Oliveira, N.D. Cardozo, C.I. Czimczik, T.R. Feldpausch, M.A. Freitas, E.
Gloor, N. Higuchi, E. Jimenez, G. Lloyd, P. Meir, C. Mendoza, A. Morel, D.A. Neill,
D. Nepstad, S. Patino, M.C. Penuela, A. Prieto, F. Ramirez, M. Schwarz, J. Silva,
M. Silveira, A.S. Thomas, H. ter Steege, J. Stropp, R. Vasquez, P. Zelazowski, E.A.
Davila, S. Andelman, A. Andrade, K.J. Chao, T. Erwin, A. Di Fiore, E. Honorio, H.
Keeling, T.J. Killeen, W.F. Laurance, A.P. Cruz, N.C.A. Pitman, P.N. Vargas, H.
Ramirez-Angulo, A. Rudas, R. Salamao, N. Silva, J. Terborgh, and A. Torres-
Lezama, 2009: Drought sensitivity of the Amazon rainforest. Science,
323(5919), 1344-1347.
Phillips, O.L., G. van der Heijden, S.L. Lewis, G. Lopez-Gonzalez, L.E.O.C. Aragao, J.
Lloyd, Y. Malhi, A. Monteagudo, S. Almeida, E. Alvarez Davila, I. Amaral, S.
Andelman, A. Andrade, L. Arroyo, G. Aymard, T.R. Baker, L. Blanc, D. Bonal, A.C.
Alves de Oliveira, K.-J. Chao, N. Davila Cardozo, L. da Costa, T.R. Feldpausch,
J.B. Fisher, N.M. Fyllas, M.A. Freitas, D. Galbraith, E. Gloor, N. Higuchi, E. Honorio,
E. Jimenez, H. Keeling, T.J. Killeen, J.C. Lovett, P. Meir, C. Mendoza, A. Morel, P.
Nunez Vargas, S. Patino, K.S.H. Peh, A. Pena Cruz, A. Prieto, C.A. Quesada, F.
Ramirez, H. Ramirez, A. Rudas, R. Salamao, M. Schwarz, J. Silva, M. Silveira,
J.W.F. Slik, B. Sonke, A.S. Thomas, J. Stropp, J.R.D. Taplin, R. Vasquez, and E.
Vilanova, 2010: Drought-mortality relationships for tropical forests. New
Phytologist, 187(3), 631-646.

351
Terrestrial and Inland Water Systems Chapter 4
4
Pielke, R.A., A. Pitman, D. Niyogi, R. Mahmood, C. McAlpine, F. Hossain, K.K. Goldewijk,
U. Nair, R. Betts, S. Fall, M. Reichstein, P. Kabat, and N. de Noblet, 2011: Land
use/land cover changes and climate: modeling analysis and observational
evidence. Wiley Interdisciplinary Reviews: Climate Change, 2(6), 828-850.
Pitman, A.J., G.T. Narisma, and J. McAneney, 2007: The impact of climate change on the
risk of forest and grassland fires in Australia. Climatic Change, 84(3-4), 383-401.
Pitman, A.J., N. de Noblet-Ducoudre, F.T. Cruz, E.L. Davin, G.B. Bonan, V. Brovkin, M.
Claussen, C. Delire, L. Ganzeveld, V. Gayler, B.J.J.M. van den Hurk, P.J. Lawrence,
M.K. van der Molen, C. Muller, C.H. Reick, S.I. Seneviratne, B.J. Strengers, and
A. Voldoire, 2009: Uncertainties in climate responses to past land cover change:
first results from the LUCID intercomparison study. Geophysical Research
Letters, 36(14), L14814, doi:10.1029/2009GL039076.
Plevin, R.J., M. O’Hare, A.D. Jones, M.S. Torn, and H.K. Gibbs, 2010: Greenhouse gas
emissions from biofuels’ indirect land use change are uncertain but may be
much greater than previously estimated. Environmental Science & Technology,
44(21), 8015-8021.
Poff, N.L., B.D. Richter, A.H. Arthington, S.E. Bunn, R.J. Naiman, E. Kendy, M. Acreman,
C. Apse, B.P. Bledsoe, M.C. Freeman, J. Henriksen, R.B. Jacobson, J.G. Kennen,
D.M. Merritt, J.H. O’Keeffe, J.D. Olden, K. Rogers, R.E. Tharme, and A. Warner,
2010: The ecological limits of hydrologic alteration (ELOHA): a new framework
for developing regional environmental flow standards. Freshwater Biology,
55(1), 147-170.
Polis, G.A., W.B. Anderson, and R.D. Holt, 1997: Toward an integration of landscape
and food web ecology: the dynamics of spatially subsidized food webs. Annual
Review of Ecology and Systematics, 28, 289-316.
Ponniah, M. and J.M. Hughes, 2004: The evolution of Queensland spiny mountain
crayfish of the genus Euastacus. I. Testing vicariance and dispersal with
interspecific mitochondrial DNA. Evolution, 58(5), 1073-1085.
Porter, T.J. and M.F.J. Pisaric, 2011: Temperature-growth divergence in white spruce
forests of Old Crow Flats, Yukon Territory, and adjacent regions of northwestern
North America. Global Change Biology, 17(11), 3418-3430.
Post, E. and J. Brodie, 2012: Extinction risk at high latitudes. In: Saving a Million
Species: Extinction Risk From Climate Change [Hannah, L. (ed.)]. Island Press,
Washington, DC, USA, pp. 121-137.
Post, E., C. Pedersen, C.C. Wilmers, and M.C. Forchhammer, 2008: Warming, plant
phenology and the spatial dimension of trophic mismatch for large herbivores.
Proceedings of the Royal Society B, 275(1646), 2005-2013.
Post, E., M.C. Forchhammer, M.S. Bret-Harte, T.V. Callaghan, T.R. Christensen, B.
Elberling, A.D. Fox, O. Gilg, D.S. Hik, T.T. Hoye, R.A. Ims, E. Jeppesen, D.R. Klein,
J. Madsen, A.D. McGuire, S. Rysgaard, D.E. Schindler, I. Stirling, M.P. Tamstorf,
N.J.C. Tyler, R. van der Wal, J. Welker, P.A. Wookey, N.M. Schmidt, and P. Aastrup,
2009: Ecological dynamics across the Arctic associated with recent climate
change. Science, 325(5946), 1355-1358.
Potts, S.G., J.C. Biesmeijer, C. Kremen, P. Neumann, O. Schweiger, and W.E. Kunin,
2010: Global pollinator declines: trends, impacts and drivers. Trends in Ecology
& Evolution, 25(6), 345-353.
Potvin, C., F. Chapin, A. Gonzalez, P. Leadley, P. Reich, and J. Roy, 2007: Plant
biodiversity and responses to elevated carbon dioxide. In: Terrestrial Ecosystems
in a Changing World [Canadell, J.G., D.E. Pataki, and L.F. Pitelka (eds.)]. Global
Change – The IGBP Series, Springer-Verlag, Berlin, Heidelberg, Germany, pp.
103-112.
Pounds, J.A., M.R. Bustamante, L.A. Coloma, J.A. Consuegra, M.P.L. Fogden, P.N.
Foster, E. La Marca, K.L. Masters, A. Merino-Viteri, R. Puschendorf, S.R. Ron, G.A.
Sanchez-Azofeifa, C.J. Still, and B.E. Young, 2006: Widespread amphibian
extinctions from epidemic disease driven by global warming. Nature,
439(7073), 161-167.
Powell, T.L., D.R. Galbraith, B.O. Christoffersen, A. Harper, H.M.A. Imbuzeiro, L. Rowland,
S. Almeida, P.M. Brando, A. Carlos Lola da Costa, M. Heil Costa, N.M. Levine, Y.
Malhi, S.R. Saleska, E. Sotta, M. Williams, P. Meir, and P.R. Moorcroft, 2013:
Confronting model predictions of carbon fluxes with measurements of Amazon
forests subjected to experimental drought. New Phytologist, 200, 350-364.
Power, S.A., E.R. Green, C.G. Barker, J.N.B. Bell, and M.R. Ashmore, 2006: Ecosystem
recovery: heathland response to a reduction in nitrogen deposition. Global
Change Biology, 12(7), 1241-1252.
Prentice, I.C. and S.P. Harrison, 2009: Ecosystem effects of CO
2
concentration:
evidence from past climates. Climate of the Past, 5(3), 297-307.
Prentice, I.C., J. Guiot, B. Huntley, D. Jolly, and R. Cheddadi, 1996: Reconstructing
biomes from palaeoecological data: a general method and its application to
European pollen data at 0 and 6 ka. Climate Dynamics, 12, 185-194.
Prentice, I.C., S.P. Harrison, and P.J. Bartlein, 2011: Global vegetation and terrestrial
carbon cycle changes after the last ice age. New Phytologist, 189(4), 988-
998.
Prieto, P., J. Penuelas, J. Llusia, D. Asensio, and M. Estiarte, 2009: Effects of long-term
experimental night-time warming and drought on photosynthesis, Fv/Fm and
stomatal conductance in the dominant species of a Mediterranean shrubland.
Acta Physiologiae Plantarum, 31(4), 729-739.
Primack, R.B., I. Ibáñez, H. Higuchi, S.D. Lee, A.J. Miller-Rushing, A.M. Wilson, and
J.A. Silander Jr., 2009: Spatial and interspecific variability in phenological
responses to warming temperatures. Biological Conservation, 142(11), 2569-
2577.
Prince, S.D., K.J. Wessels, C.J. Tucker, and S.E. Nicholson, 2007: Desertification in the
Sahel: a reinterpretation of a reinterpretation. Global Change Biology, 13(7),
1308-1313.
Pringle, C.M., 2001: Hydrologic connectivity and the management of biological
reserves: a global perspective. Ecological Applications, 11(4), 981-998.
Prost, S., R.P. Guralnick, E. Waltari, V.B. Fedorov, E. Kuzmina, N. Smirnov, T. Van
Kolfschoten, M. Hofreiter, and K. Vrieling, 2013: Losing ground: past history and
future fate of Arctic small mammals in a changing climate. Global Change
Biology, 19(6), 1854-1864.
Prowse, T.D. and K. Brown, 2010: Hydro-ecological effects of changing Arctic river
and lake ice covers: a review. Hydrology Research, 41(6), 454-461.
Prugh, L.R., K.E. Hodges, A.R.E. Sinclair, and J.S. Brashares, 2008: Effect of habitat
area and isolation on fragmented animal populations. Proceedings of the
National Academy of Sciences of the United States of America, 105(52), 20770-
20775.
Pulido, F., 2007: Phenotypic changes in spring arrival: evolution, phenotypic plasticity,
effects of weather and condition. Climate Research, 35(1-2), 5-23.
Racine, C., R. Jandt, C. Meyers, and J. Dennis, 2004: Tundra fire and vegetation change
along a hillslope on the Seward Peninsula, Alaska, U.S.A. Arctic, Antarctic, and
Alpine Research, 36(1), 1-10.
Raffa, K.F., B.H. Aukema, B.J. Bentz, A.L. Carroll, J.A. Hicke, M.G. Turner, and
W.H. Romme, 2008: Cross-scale drivers of natural disturbances prone to
anthropogenic amplification: the dynamics of bark beetle eruptions. BioScience,
58(6), 501-517.
Raghu, S., R.C. Anderson, C.C. Daehler, A.S. Davis, R.N. Wiedenmann, D. Simberloff,
and R.N. Mack, 2006: Adding biofuels to the invasive species fire? Science,
313(5794), 1742-1742.
Rahel, F.J. and J.D. Olden, 2008: Assessing the effects of climate change on aquatic
invasive species. Conservation Biology, 22(3), 521-533.
Randerson, J.T., H. Liu, M.G. Flanner, S.D. Chambers, Y. Jin, P.G. Hess, G. Pfister, M.C.
Mack, K.K. Treseder, L.R. Welp, F.S. Chapin, J.W. Harden, M.L. Goulden, E. Lyons,
J.C. Neff, E.A.G. Schuur, and C.S. Zender, 2006: The impact of boreal forest fire
on climate warming. Science, 314(5802), 1130-1132.
Randin, C.F., R. Engler, S. Normand, M. Zappa, N.E. Zimmermann, P.B. Pearman, P.
Vittoz, W. Thuiller, and A. Guisan, 2009: Climate change and plant distribution:
local models predict high-elevation persistence. Global Change Biology, 15(6),
1557-1569.
Raupach, M.R., J.G. Canadell, and C. Le Quéré, 2008: Anthropogenic and biophysical
contributions to increasing atmospheric CO
2
growth rate and airborne fraction.
Biogeosciences, 5(6), 1601-1613.
Ravi, S., D.D. Breshears, T.E. Huxman, and P. D’Odorico, 2010: Land degradation in
drylands: interactions among hydrologic-aeolian erosion and vegetation
dynamics. Geomorphology, 116(3-4), 236-245.
Rawson, D.M., G.M. Reid, and R.E. Lloyd, 2011: Conservation rationale, research
applications and techniques in the cryopreservation of lower vertebrate
biodiversity from marine and freshwater environments. International Zoo
Yearbook, 45, 108-123.
Raxworthy, C.J., R.G. Pearson, N. Rabibisoa, A.M. Rakotondrazafy, J.B. Ramanamanjato,
A.P. Raselimanana, S. Wu, R.A. Nussbaum, and D.A. Stone, 2008: Extinction
vulnerability of tropical montane endemism from warming and upslope
displacement: a preliminary appraisal for the highest massif in Madagascar.
Global Change Biology, 14(8), 1703-1720.
Ray, D., D. Nepstad, and P. Moutinho, 2005: Micrometeorological and canopy controls
of flammability in mature and disturbed forests in an east-central Amazon
landscape. Ecological Applications, 15(5), 1664-1678.
Ray, D.K., N. Ramankutty, N.D. Mueller, P.C. West, and J.A. Foley, 2012: Recent
patterns of crop yield growth and stagnation. Nature Communications, 3, 1293,
doi:10.1038/ncomms2296.

352
Chapter 4 Terrestrial and Inland Water Systems
4
Réale, D., A.G. McAdam, S. Boutin, and D. Berteaux, 2003: Genetic and plastic
responses of a northern mammal to climate change. Proceedings of the Royal
Society B, 270(1515), 591-596.
Reed, T.E., V. Grotan, S. Jenouvrier, B.E. Saether, and M.E. Visser, 2013: Population
growth in a wild bird is buffered against phenological mismatch. Science,
340(6131), 488-491.
Regnier, C., B. Fontaine, and P. Bouchet, 2009: Not knowing, not recording, not
listing: numerous unnoticed mollusk extinctions. Conservation Biology, 23(5),
1214-1221.
Rehfeldt, G.E. and B.C. Jaquish, 2010: Ecological impacts and management strategies
for western larch in the face of climate-change. Mitigation and Adaptation
Strategies for Global Change, 15(3), 283-306.
Rehfeldt, G.E., N.L. Crookston, C. Saenz-Romero, and E.M. Campbell, 2012: North
American vegetation model for land-use planning in a changing climate: a
solution to large classification problems. Ecological Applications, 22(1), 119-141.
Reich, P.B., 2009: Elevated CO
2
reduces losses of plant diversity caused by nitrogen
deposition. Science, 326(5958), 1399-1402.
Reich, P.B., S.E. Hobbie, T. Lee, D.S. Ellsworth, J.B. West, D. Tilman, J.M.H. Knops, S.
Naeem, and J. Trost, 2006: Nitrogen limitation constrains sustainability of
ecosystem response to CO
2
. Nature, 440(7086), 922-925.
Reidy Liermann, C., C. Nilsson, J. Robertson, and R.Y. Ng, 2012: Implications of dam
obstruction for global freshwater fish diversity. BioScience, 62(6), 539-548.
Reist, J.D., F.J. Wrona, T.D. Prowse, M. Power, J.B. Dempson, R.J. Beamish, J.R. King,
T.J. Carmichael, and C.D. Sawatzky, 2006: General effects of climate change on
Arctic fishes and fish populations. Ambio, 35(7), 370-380.
Renwick, A.R., D. Massimino, S.E. Newson, D.E. Chamberlain, J.W. Pearce-Higgins,
and A. Johnston, 2012: Modelling changes in species’ abundance in response
to projected climate change. Diversity and Distributions, 18(2), 121-132.
Riahi, K., S. Rao, V. Krey, C. Cho, V. Chirkov, G. Fischer, G. Kindermann, N. Nakicenovic,
and P. Rafaj, 2011: RCP 8.5 – a scenrio of comparatively high greenhouse gas
emissions. Climatic Change, 109, 33-57.
Ricciardi, A. and D. Simberloff, 2009: Assisted colonization is not a viable conservation
strategy. Trends in Ecology & Evolution, 24(5), 248-253.
Richardson, D.M., J.J. Hellmann, J.S. McLachlan, D.F. Sax, M.W. Schwartz, P. Gonzalez,
E.J. Brennan, A. Camacho, T.L. Root, O.E. Sala, S.H. Schneider, D.M. Ashe, J.R.
Clark, R. Early, J.R. Etterson, E.D. Fielder, J.L. Gill, B.A. Minteer, S. Polasky, H.D.
Safford, A.R. Thompson, and M. Vellend, 2009: Multidimensional evaluation of
managed relocation. Proceedings of the National Academy of Sciences of the
United States of America, 106(24), 9721-9724.
Ridgwell, A., J.S. Singarayer, A.M. Hetherington, and P.J. Valdes, 2009: Tackling
regional climate change by leaf albedo bio-geoengineering. Current Biology,
19(2), 146-150.
Rieley, J.O., R.A.J. Wüst, J. Jauhiainen, S.E. Page, J.H.M. Wösten, A. Hooijer, E. Siegert,
S.H. Limin, H. Vasander, and M. Stahlhut, 2008: Tropical peatlands: carbon
stores, carbon gas emissions and contribution to climate change processes. In:
Peatlands and Climate Change [Strack, M. (ed.)]. International Peat Society,
Jyväskylä, Finland, pp. 148-181.
Roberts, S.P.M., S.G. Potts, J. Biesmeijer, M. Kuhlmann, B. Kunin, and R. Ohlemüller,
2011: Assessing continental-scale risks for generalist and specialist pollinating
bee species under climate change. BioRisk, 6, 1-18.
Robinet, C. and A. Roques, 2010: Direct impacts of recent climate warming on insect
populations. Integrative Zoology, 5(2), 132-142.
Rocha, A.V. and G.R. Shaver, 2011: Burn severity influences postfire CO
2
exchange in
arctic tundra. Ecological Applications, 21(2), 477-489.
Rodriguez-Labajos, B., 2013: Climate and change, ecosystems services and costs
of action and inaction: scoping the interface. Wiley Interdisciplinary Review:
Climate Change, 4(6), 555-573.
Rohde, R.F. and M.T. Hoffman, 2012: The historical ecology of Namibian rangelands:
Vegetation change since 1876 in response to local and global drivers.Science
of the Total Environment, 416, 276-288.
Roland, C.A., J.H. Schmidt, and E.F. Nicklen, 2013: Landscape-scale patterns in tree
occupancy and abundance in subarctic Alaska. Ecological Monographs, 83(1),
19-48.
Romanovsky, V.E., S.L. Smith, and H.H. Christiansen, 2010: Permafrost thermal state
in the polar Northern Hemisphere during the International Polar Year 2007-
2009: a synthesis. Permafrost and Periglacial Processes, 21(2), 106-116.
Romijn, E., M. Herold, L. Kooistra, D. Murdiyarso, and L. Verchot, 2012: Assessing
capacities of non-Annex I countries for national forest monitoring in the context
of REDD+. Environmental Science and Policy, 19-20, 33-48.
Root, T.L., J.T. Price, K.R. Hall, S.H. Schneider, C. Rosenzweig, and J.A. Pounds, 2003:
Fingerprints of global warming on wild animals and plants. Nature, 421(6918),
57-60.
Rosenheim, J.A. and B.E. Tabashnik, 1991: Influence of generation time on the
response to selection. American Naturalist, 137(4), 527-541.
Rosenzweig, C., G. Casassa, D.J. Karoly, A. Imeson, C. Liu, A. Menzel, S. Rawlins, T.L.
Root, B. Seguin, and P. Tryjanowski, 2007: Assessment of observed changes and
responses in natural and managed systems. In: Climate Change 2007: Impacts,
Adaptation and Vulnerability. Contribution of Working Group II to the Fourth
Assessment Report of the Intergovernmental Panel on Climate Change [Parry,
M.L., O.F. Canziani, J.P. Palutikof, P.J. van der Linden, and C.E. Hanson (eds.)].
Cambridge University Press, Cambridge, UK and New York, NY, USA, pp. 79-131.
Rosset, V., A. Lehmann, and B. Oertli, 2010: Warmer and richer? Predicting the impact
of climate warming on species richness in small temperate waterbodies. Global
Change Biology, 16(8), 2376-2387.
Rössler, M., 2006: World heritage cultural landscapes: a UNESCO flagship
programme 1992-2006. Landscape Research, 31(4), 333-353.
Rounsevell, M.D.A. and D.S. Reay, 2009: Land use and climate change in the UK.
Land Use Policy, 26(Suppl 1), S160-S169.
Roux, D.J., J.L. Nel, P.J. Ashton, A.R. Deaconc, F.C. de Moor, D. Hardwick, L. Hill, C.J.
Kleynhans, G.A. Maree, J. Moolman, and R.J. Scholes, 2008: Designing protected
areas to conserve riverine biodiversity: lessons from a hypothetical redesign of
the Kruger National Park. Biological Conservation, 141(1), 100-117.
Rowe, R.J., J.A. Finarelli, and E.A. Rickart, 2010: Range dynamics of small mammals
along an elevational gradient over an 80-year interval. Global Change Biology,
16(11), 2930-2943.
Rubidge, E.M., W.B. Monahan, J.L. Parra, S.E. Cameron, and J.S. Brashares, 2011: The
role of climate, habitat, and species co-occurrence as drivers of change in small
mammal distributions over the past century. Global Change Biology, 17(2),
696-708.
Ruiz-Labourdette, D., M.F. Schmitz, and F.D. Pineda, 2013: Changes in tree species
composition in Mediterranean mountains under climate change: indicators for
conservation planning. Ecological Indicators, 24, 310-323.
Rupp, T.S., F.S. Chapin, and A. Starfield, 2001: Modeling the influence of topographic
barriers on treeline advance at the forest-tundra ecotone in northwestern
Alaska. Climatic Change, 48(2), 399-416.
Russell, L.M., P.J. Rasch, G.M. Mace, R.B. Jackson, J. Shepherd, P. Liss, M. Leinen, D.
Schimel, N.E. Vaughan, A.C. Janetos, P.W. Boyd, R.J. Norby, K. Caldeira, J.
Merikanto, P. Artaxo, J. Melillo, and M.G. Morgan, 2012: Ecosystem impacts of
geoengineering: a review for developing a science plan. Ambio, 41(4), 350-
369.
Rustad, L.E., 2008: The response of terrestrial ecosystems to global climate change:
towards an integrated approach. Science of the Total Environment, 404(2-3),
222-235.
Ryan, M.G., M.E. Harmon, R.A. Birdsey, C.P. Giardina, L.S. Heath, R.A. Houghton, R.B.
Jackson, D.C. McKinley, J.F. Morrison, B.C. Murray, D.E. Pataki, and K.E. Skog,
2010: A synthesis of the science on forests and carbon for U.S. forests. Issues
in Ecology, 13, 1-16.
Saatchi, S., S. Asefi-Najafabady, Y. Malhi, L.E.O.C. Aragão, L.O. Anderson, R.B. Myneni,
and R. Nemani, 2013: Persistent effects of a severe drought on Amazonian
forest canopy. Proceedings of the National Academy of Sciences of the United
States of America, 110(2), 565-570.
Saino, N., D. Rubolini, E. Lehikoinen, L.V. Sokolov, A. Bonisoli-Alquati, R. Ambrosini,
G. Boncoraglio, and A.P. Moller, 2009: Climate change effects on migration
phenology may mismatch brood parasitic cuckoos and their hosts. Biology
Letters, 5(4), 539-541.
Sala, O.E., W.J. Parton, L.A. Joyce, and W.K. Lauenroth, 1988: Primary production of
the Central Grassland Region of the United States. Ecology, 69(1), 10-45.
Salamin, N., R.O. Wuest, S. Lavergne, W. Thuiller, and P.B. Pearman, 2010: Assessing
rapid evolution in a changing environment. Trends in Ecology & Evolution,
25(12), 692-698.
Salzmann, U., A.M. Haywood, D.J. Lunt, P.J. Valdes, and D.J. Hill, 2008: A new global
biome reconstruction and data-model comparison for the Middle Pliocene.
Global Ecology and Biogeography, 17(3), 432-447.
Samanta, A., M.H. Costa, E.L. Nunes, S.A. Vieira, L. Xu, and R.B. Myneni, 2011: Comment
on “Drought-induced reduction in global terrestrial net primary production
from 2000 through 2009”. Science, 333(6046), 1093-c.
Sandel, B. and E.M. Dangremond, 2012: Climate change and the invasion of California
by grasses. Global Change Biology, 18(1), 277-289.

353
Terrestrial and Inland Water Systems Chapter 4
4
Sandel, B., L. Arge, B. Dalsgaard, R.G. Davies, K.J. Gaston, W.J. Sutherland, and J.C.
Svenning, 2011: The influence of Late Quaternary climate-change velocity on
species endemism. Science, 334(6056), 660-664.
Sankaran, M., N.P. Hanan, R.J. Scholes, J. Ratnam, D.J. Augustine, B.S. Cade, J. Gignoux,
S.I. Higgins, X. Le Roux, F. Ludwig, J. Ardo, F. Banyikwa, A. Bronn, G. Bucini, K.K.
Caylor, M.B. Coughenour, A. Diouf, W. Ekaya, C.J. Feral, E.C. February, P.G.H.
Frost, P. Hiernaux, H. Hrabar, K.L. Metzger, H.H.T. Prins, S. Ringrose, W. Sea, J.
Tews, J. Worden, and N. Zambatis, 2005: Determinants of woody cover in African
savannas. Nature, 438(7069), 846-849.
Santini, L., M. Di Marco, P. Visconti, D. Baisero, L. Boitani, and C. Rondinini, 2013:
Ecological correlates of dispersal distance in terrestrial mammals. Hystrix, the
Italian Journal of Mammalogy, 24(2), doi:10.4404/hystrix-24.2-8746.
Saraux, C., C. Le Bohec, J.M. Durant, V.A. Viblanc, M. Gauthier-Clerc, D. Beaune, Y.-H.
Park, N.G. Yoccoz, N.C. Stenseth, and Y. Le Maho, 2011: Reliability of flipper-
banded penguins as indicators of climate change. Nature, 469(7329), 203-206.
Sardans, J., J. Penuelas, M. Estiarte, and P. Prieto, 2008a: Warming and drought alter
C and N concentration, allocation and accumulation in a Mediterranean
shrubland. Global Change Biology, 14(10), 2304-2316.
Sardans, J., J. Penuelas, P. Prieto, and M. Estiarte, 2008b: Changes in Ca, Fe, Mg, Mo,
Na, and S content in a Mediterranean shrubland under warming and drought.
Journal of Geophysical Research: Biogeosciences, 113(G3), G03039, doi:10.1029/
2008JG000795.
Sardans, J., A. Rivas-Ubach, and J. Penuelas, 2012: The C:N:P stoichiometry of organisms
and ecosystems in a changing world: a review and perspectives. Perspectives
in Plant Ecology Evolution and Systematics, 14(1), 33-47.
Sarris, D., D. Christodoulakis, and C. Körner, 2011: Impact of recent climatic change
on growth of low elevation eastern Mediterranean forest trees. Climatic
Change, 106(2), 203-223.
Sato, H. and T. Ise, 2012: Effect of plant dynamic processes on African vegetation
responses to climate change: analysis using the spatially explicit individual-
based dynamic global vegetation model (SEIB-DGVM). Journal of Geophysical
Research: Biogeosciences, 117(G3), G03017, doi:10.1029/2012JG002056.
Sauer, J., S. Domisch, C. Nowak, and P. Haase, 2011: Low mountain ranges: summit
traps for montane freshwater species under climate change. Biodiversity and
Conservation, 20(13), 3133-3146.
Saurral, R.I., V.R. Barros, and D.P. Lettenmaier, 2008: Land use impact on the Uruguay
River discharge. Geophysical Research Letters, 35(12), L12401.
Schaefer, K., T.J. Zhang, L. Bruhwiler, and A.P. Barrett, 2011: Amount and timing of
permafrost carbon release in response to climate warming. Tellus Series B:
Chemical and Physical Meteorology, 63(2), 165-180.
Schaper, S.V., A. Dawson, P.J. Sharp, P. Gienapp, S.P. Caro, and M.E. Visser, 2012:
Increasing temperature, not mean temperature, is a cue for avian timing of
reproduction. The American Naturalist, 179(2), E55-E69.
Scheffer, M., 2009: Critical Transitions in Nature and Society. Princeton University
Press, Princeton, NJ, USA, 400 pp.
Scheffer, M., J. Bascompte, W.A. Brock, V. Brovkin, S.R. Carpenter, V. Dakos, H. Held,
E.H. van Nes, M. Rietkerk, and G. Sugihara, 2009: Early-warning signals for
critical transitions. Nature, 461(7260), 53-59.
Scheffer, M., M. Hirota, M. Holmgren, E.H. Van Nes, and F.S. Chapin III, 2012: Thresholds
for boreal biome transitions. Proceedings of the National Academy of Sciences
of the United States of America, 109(52), 21384-21389.
Scheiter, S. and S.I. Higgins, 2009: Impacts of climate change on the vegetation of
Africa: an adaptive dynamic vegetation modelling approach. Global Change
Biology, 15(9), 2224-2246.
Schiffers, K., E.C. Bourne, S. Lavergne, W. Thuiller, and J.M.J. Travis, 2013: Limited
evolutionary rescue of locally adapted populations facing climate change.
Philosophical Transactions of the Royal Society B, 368(1610), 20120083,
doi:10.1098/rstb.2012.0083.
Schippers, P., J. Verboom, C.C. Vos, and R. Jochem, 2011: Metapopulation shift and
survival of woodland birds under climate change: will species be able to track?
Ecography, 34(6), 909-919.
Schloss, C.A., T.A. Nunez, and J.J. Lawler, 2012: Dispersal will limit ability of mammals
to track climate change in the Western Hemisphere. Proceedings of the National
Academy of Sciences of the United States of America, 109(22), 8606-8611.
Schneider, A., M.A. Friedl, and D. Potere, 2009: A new map of global urban extent
from MODIS satellite data. Environmental Research Letters, 4(4), 044003,
doi:10.1088/1748-9326/4/4/044003.
Schneider, C., 2003: The influence of spatial scale on quantifying insect dispersal:
an analysis of butterfly data. Ecological Entomology, 28(2), 252-256.
Schnitzler, A., B.W. Hale, and E.M. Alsum, 2007: Examining native and exotic species
diversity in European riparian forests. Biological Conservation, 138(1-2), 146-
156.
Scholes, R.J. and S.R. Archer, 1997: Tree-grass interactions in savannas. Annual
Review of Ecology and Systematics, 28, 517-544.
Scholze, M., W. Knorr, N.W. Arnell, and I.C. Prentice, 2006: A climate-change risk
analysis for world ecosystems. Proceedings of the National Academy of
Sciences of the United States of America, 103(35), 13116-13120.
Schulte, P., L. Alegret, I. Arenillas, J.A. Arz, P.J. Barton, P.R. Bown, T.J. Bralower, G.L.
Christeson, P. Claeys, C.S. Cockell, G.S. Collins, A. Deutsch, T.J. Goldin, K. Goto,
J.M. Grajales-Nishimura, R.A.F. Grieve, S.P.S. Gulick, K.R. Johnson, W. Kiessling,
C. Koeberl, D.A. Kring, K.G. MacLeod, T. Matsui, J. Melosh, A. Montanari, J.V.
Morgan, C.R. Neal, D.J. Nichols, R.D. Norris, E. Pierazzo, G. Ravizza, M.
Rebolledo-Vieyra, W.U. Reimold, E. Robin, T. Salge, R.P. Speijer, A.R. Sweet, J.
Urrutia-Fucugauchi, V. Vajda, M.T. Whalen, and P.S. Willumsen, 2010: The
Chicxulub Asteroid impact and mass extinction at the Cretaceous-Paleogene
Boundary. Science, 327(5970), 1214-1218.
Schultz, M.G., A. Heil, J.J. Hoelzemann, A. Spessa, K. Thonicke, J.G. Goldammer, A.C.
Held, J.M.C. Pereira, and M. van het Bolscher, 2008: Global wildland fire
emissions from 1960 to 2000. Global Biogeochemical Cycles, 22(2), GB2002,
doi:10.1029/2007GB003031.
Schuur, E.A.G., J. Bockheim, J.G. Canadell, E. Euskirchen, C.B. Field, S.V. Goryachkin,
S. Hagemann, P. Kuhry, P.M. Lafleur, H. Lee, G. Mazhitova, F.E. Nelson, A. Rinke,
V.E. Romanovsky, N. Shiklomanov, C. Tarnocai, S. Venevsky, J.G. Vogel, and S.A.
Zimov, 2008: Vulnerability of permafrost carbon to climate change: implications
for the global carbon cycle. BioScience, 58(8), 701-714.
Schuur, E.A.G., J.G. Vogel, K.G. Crummer, H. Lee, J.O. Sickman, and T.E. Osterkamp,
2009: The effect of permafrost thaw on old carbon release and net carbon
exchange from tundra. Nature, 459(7246), 556-559.
Schuur, E.A.G., B.W. Abbott, W.B. Bowden, V. Brovkin, P. Camill, J.G. Canadell, J.P.
Chanton, F.S. Chapin III, T.R. Christensen, P. Ciais, B.T. Crosby, C.I. Czimczik, G.
Grosse, J. Harden, D.J. Hayes, G. Hugelius, J.D. Jastrow, J.B. Jones, T. Kleinen, C.D.
Koven, G. Krinner, P. Kuhry, D.M. Lawrence, A.D. McGuire, S.M. Natali, J.A.
O’Donnell, C.L. Ping, W.J. Riley, A. Rinke, V.E. Romanovsky, A.B.K. Sannel, C.
Schädel, K. Schaefer, J. Sky, Z.M. Subin, C. Tarnocai, M.R. Turetsky, M.P. Waldrop,
K.M. Walter Anthony, K.P. Wickland, C.J. Wilson, and S.A. Zimov, 2013: Expert
assessment of vulnerability of permafrost carbon to climate change. Climate
Change, 119, 359–374. doi:10.1007/s10584-013-0730-7.
Schwaiger, H.P. and D.N. Bird, 2010: Integration of albedo effects caused by land
use change into the climate balance: should we still account in greenhouse
gas units? Forest Ecology and Management, 260(3), 278-286.
Schweiger, O., J. Settele, O. Kudrna, S. Klotz, and I. Kühn, 2008: Climate change can
cause spatial mismatch of trophically interacting species. Ecology, 89(12),
3472-3479.
Schweiger, O., J.C. Biesmeijer, R. Bommarco, T. Hickler, P.E. Hulme, S. Klotz, I. Kuehn, M.
Moora, A. Nielsen, R. Ohlemüller, T. Petanidou, S.G. Potts, P. Pyšek, J.C. Stout, M.T.
Sykes, T. Tscheulin, M. Vila, G.-R. Walther, C. Westphal, M. Winter, M. Zobel, and J.
Settele, 2010: Multiple stressors on biotic interactions: how climate change and
alien species interact to affect pollination. Biological Reviews, 85(4), 777-795.
Schweiger, O., A. Harpke, R. Heikkinen, T. Hickler, I. Kühn, J. Pöyry, and J. Settele,
2012: Increasing range mismatching of interacting species under global change
is related to their ecological characteristics. Global Ecology and Biogeography,
21(1), 88-99.
Seaquist, J.W., T. Hickler, L. Eklundh, J. Ardö, and B.W. Heumann, 2009: Disentangling
the effects of climate and people on Sahel vegetation dynamics. Biogeosciences,
6(3), 469-477.
Searchinger, T., R. Heimlich, R.A. Houghton, F.X. Dong, A. Elobeid, J. Fabiosa, S.
Tokgoz, D. Hayes, and T.H. Yu, 2008: Use of US croplands for biofuels increases
greenhouse gases through emissions from land-use change. Science,
319(5867), 1238-1240.
Seidel, D.J., Q. Fu, W.J. Randel, and T.J. Reichler, 2008: Widening of the tropical belt
in a changing climate. Nature Geoscience, 1(1), 21-24.
Sekercioglu, C.H., R.B. Primack, and J. Wormworth, 2012: The effects of climate
change on tropical birds. Biological Conservation, 148(1), 1-18.
Selsted, M.B., L. van der Linden, A. Ibrom, A. Michelsen, K.S. Larsen, J.K. Pedersen,
T.N. Mikkelsen, K. Pilegaard, C. Beier, and P. Ambus, 2012: Soil respiration is
stimulated by elevated CO
2
and reduced by summer drought: three years of
measurements in a multifactor ecosystem manipulation experiment in a
temperate heathland (CLIMAITE). Global Change Biology, 18(4), 1216-1230.

354
Chapter 4 Terrestrial and Inland Water Systems
4
Seppälä, R., 2009: A global assessment on adaptation of forests to climate change.
Scandinavian Journal of Forest Research, 24(6), 469-472.
Seppelt, R., C.F. Dormann, F.V. Eppink, S. Lautenbach, and S. Schmidt, 2011: A
quantitative review of ecosystem service studies: approaches, shortcomings
and the road ahead. Journal of Applied Ecology, 48(3), 630-636.
Serreze, M.C. and J.A. Francis, 2006: The Arctic amplification debate. Climate Change,
76(3), 241-264.
Settele, J. and E. Kühn, 2009: Insect conservation. Science, 325(5936), 41-42.
Settele, J., O. Kudrna, A. Harpke, I. Kühn, C. Van Swaay, R. Verovnik, M. Warren, M.
Wiemers, J. Hanspach, T. Hickler, E. Kühn, I. Van Halder, K. Veling, A. Vliegenthart,
I. Wynhoff, and O. Schweiger, 2008: Special Issue: Climatic Risk Atlas of
European Butterflies. BioRisk, 1, 1-710, doi:10.3897/biorisk.1.
Settele, J., L. Penev, T. Georgiev, R. Grabaum, V. Grobelnik, V. Hammen, S. Klotz, M.
Kotarac, and I. Kühn (eds.), 2010a: Atlas of Biodiversity Risk. Pensoft Publishers,
Sofia, Bulgaria, 300 pp.
Settele, J., M. Zobel, J.H. Spangenberg, S. Klotz, V. Hammen, and I. Kühn, 2010b:
Designing projects for integrated research – the ALARM experience. In: Atlas
of Biodiversity Risk [Settele, J., L. Penev, T. Georgiev, R. Grabaum, V. Grobelnik,
V. Hammen, S. Klotz, M. Kotarac, and I. Kühn (eds.)]. Pensoft Publishers, Sofia,
Bulgaria, pp. 208-209.
Shakesby, R.A., 2011: Post-wildfire soil erosion in the Mediterranean: review and
future research directions. Earth-Science Reviews, 105(3-4), 71-100.
Shanin, V.N., A.S. Komarov, A.V. Mikhailov, and S.S. Bykhovets, 2011: Modelling carbon
and nitrogen dynamics in forest ecosystems of Central Russia under different
climate change scenarios and forest management regimes. Ecological
Modelling, 222(14), 2262-2275.
Sharma, S., S. Couturier, and S.D. Cote, 2009: Impacts of climate change on the seasonal
distribution of migratory caribou. Global Change Biology, 15(10), 2549-2562.
Sharp, B.R. and D.M.J.S. Bowman, 2004: Patterns of long-term woody vegetation
change in a sandstone-plateau savanna woodland, Northern Territory, Australia.
Journal of Tropical Ecology, 20(03), 259-270.
Shaw, M.R., E.S. Zavaleta, N.R. Chiariello, E.E. Cleland, H.A. Mooney, and C.B. Field,
2002: Grassland responses to global environmental changes suppressed by
elevated CO
2
. Science, 298(5600), 1987-1990.
Sheldon, F., S.E. Bunn, J.M. Hughes, A.H. Arthington, S.R. Balcombe, and C.S. Fellows,
2010: Ecological roles and threats to aquatic refugia in arid landscapes: dryland
river waterholes. Marine and Freshwater Research, 61(8), 885-895.
Shimazaki, M., I. Tsuyama, E. Nakazono, K. Nakao, M. Konoshima, N. Tanaka, and T.
Nakashizuka, 2012: Fine-resolution assessment of potential refugia for a
dominant fir species (Abies mariesii) of subalpine coniferous forests after
climate change. Plant Ecology, 213(4), 603-612.
Shimoda, Y., M.E. Azim, G. Perhar, M. Ramin, M.A. Kenney, S. Sadraddini, A. Gudimov,
and G.B. Arhonditsis, 2011: Our current understanding of lake ecosystem
response to climate change: what have we really learned from the north
temperate deep lakes? Journal of Great Lakes Research, 37(1), 173-193.
Shinoda, M., G.U. Nachinshonhor, and M. Nemoto, 2010: Impact of drought on
vegetation dynamics of the Mongolian steppe: a field experiment. Journal of
Arid Environments, 74(1), 63-69.
Shiogama, H., S. Emori, N. Hanasaki, M. Abe, Y. Masutomi, K. Takahashi, and T.
Nozawa, 2011: Observational constraints indicate risk of drying in the Amazon
basin. Nature Communications, 2, 253, doi:10.1038/ncomms1252.
Silva, L.C.R. and M. Anand, 2013: Probing for the influence of atmospheric CO
2
and
climate change on forest ecosystems across biomes. Global Ecology and
Biogeography, 22(1), 83-92.
Silva, L.C.R., M. Anand, and M.D. Leithead, 2010: Recent widespread tree growth
decline despite increasing atmospheric CO
2
. PLoS One, 5(7), e11543, doi:10.1371/
journal.pone.0011543.
Silvestrini, R., B. Soares-Filho, D. Nepstad, M.T. Coe, H. Rodrigues, and R. Assuncao,
2011: Simulating fire regimes in the Amazon in response to climate change
and deforestation. Ecological Applications, 21(5), 1573-1590.
Simberloff, D., J.-L. Martin, P. Genovesi, V. Maris, D.A. Wardle, J. Aronson, F. Courchamp,
B. Galil, E. García-Berthou, M. Pascal, P. Pyšek, R. Sousa, E. Tabacchi, and M.
Vilà, 2013: Impacts of biological invasions: what’s what and the way forward.
Trends in Ecology & Evolution, 28(1), 58-66.
Sinervo, B., F. Mendez-de-la-Cruz, D.B. Miles, B. Heulin, E. Bastiaans, M.V.S. Cruz, R.
Lara-Resendiz, N. Martinez-Mendez, M.L. Calderon-Espinosa, R.N. Meza-Lazaro,
H. Gadsden, L.J. Avila, M. Morando, I.J. De la Riva, P.V. Sepulveda, C.F.D. Rocha,
N. Ibarguengoytia, C.A. Puntriano, M. Massot, V. Lepetz, T.A. Oksanen, D.G.
Chapple, A.M. Bauer, W.R. Branch, J. Clobert, and J.W. Sites, 2010: Erosion of
lizard diversity by climate change and altered thermal niches. Science,
328(5980), 894-899.
Singer, M.C. and C. Parmesan, 2010: Phenological asynchrony between herbivorous
insects and their hosts: signal of climate change or pre-existing adaptive
strategy? Philosophical Transactions of the Royal Society B, 365(1555), 3161-
3176.
Singh, A., S. Unnikrishnan, N. Naik, and K. Duvvuri, 2013: Role of India´s forest in climate
change mitigation through the CDM and REDD+. Journal of Environmental
Planning and Management, 56, 61-87.
Sitch, S., P.M. Cox, W.J. Collins, and C. Huntingford, 2007: Indirect radiative forcing
of climate change through ozone effects on the land-carbon sink. Nature,
448(7155), 791-794.
Sitch, S., C. Huntingford, N. Gedney, P.E. Levy, M. Lomas, S.L. Piao, R. Betts, P. Ciais,
P. Cox, P. Friedlingstein, C.D. Jones, I.C. Prentice, and F.I. Woodward, 2008:
Evaluation of the terrestrial carbon cycle, future plant geography and climate-
carbon cycle feedbacks using five Dynamic Global Vegetation Models (DGVMs).
Global Change Biology, 14(9), 2015-2039.
Smallwood, K.S., 2007: Estimating wind turbine-caused bird mortality. Journal of
Wildlife Management, 71(8), 2781-2791.
Smit, B., I. Burton, R.J.T. Klein, and J. Wandel, 2000: An anatomy of adaptation to
climate change and variability. Climatic Change, 45(1), 223-251.
Smit, B., O. Pilifosova, I. Burton, B. Challenger, S. Huq, R.J.T. Klein, G. Yohe, N. Adger,
T. Downing, E. Harvey, S. Kane, M. Parry, M. Skinner, J. Smith, and J. Wandel,
2007: Adaptation to climate change in the context of sustainable development
and equity. In: Climate Change 2001: Impacts, Adaptation, and Vulnerability.
Contribution of Working Group II to the Third Assessment Report of the
Intergovernmental Panel on Climate Change [McCarthy, J., O. Canziani, N. Leary,
D. Dokken, and K. White (eds.)]. Cambridge University Press, Cambridge, UK
and New York, NY, USA, pp. 879-912.
Smith, A.L., N. Hewitt, N. Klenk, D.R. Bazely, N. Yan, S. Wood, I. Henriques, J.I. MacLellan, and
C. Lipsig-Mummé, 2012: Effects of climate change on the distribution of invasive
alien species in Canada: a knowledge synthesis of range change projections in
a warming world. Environmental Reviews, 20(1), doi:10.1139/a11-020.
Smith, L.C., Y. Sheng, G.M. MacDonald, and L.D. Hinzman, 2005: Disappearing Arctic
lakes. Science, 308(5727), 1429-1429.
Smith, S.J. and T.M.L. Wigley, 2006: Multi-gas forcing stabilization with minicam. The
Energy Journal, 27 (SI3), 373-391.
Smol, J.P. and M.S.V. Douglas, 2007a: From controversy to consensus: making the
case for recent climate using lake sediments. Frontiers in Ecology and the
Environment, 5(9), 466-474.
Smol, J.P. and M.S.V. Douglas, 2007b: Crossing the final ecological threshold in high
Arctic ponds. Proceedings of the National Academy of Sciences of the United
States of America, 104(30), 12395-12397.
Snyman, H.A. and H.J. Fouché, 1993: Estimating seasonal herbage production of a
semi-arid grassland based on veld condition, rainfall, and evapotranspiration.
African Journal of Range and Forage Science, 10(1), 21-24.
Soares-Filho, B., P. Moutinho, D. Nepstad, A. Anderson, H. Rodrigues, R. Garcia, L.
Dietzsch, F. Merry, M. Bowman, L. Hissa, R. Silvestrini, and C. Maretti, 2010:
Role of Brazilian Amazon protected areas in climate change mitigation.
Proceedings of the National Academy of Sciences of the United States of
America, 107(24), 10821-10826.
Soares-Filho, B., R. Silvestrini, D. Nepstad, P. Brando, H. Rodrigues, A. Alencar, M.
Coe, C. Locks, L. Lima, L. Hissa, and C. Stickler, 2012: Forest fragmentation,
climate change and understory fire regimes on the Amazonian landscapes of
the Xingu headwaters. Landscape Ecology, 27(4), 585-598.
Sobek-Swant, S., J.C. Crosthwaite, D.B. Lyons, and B.J. Sinclair, 2012: Could phenotypic
plasticity limit an invasive species? Incomplete reversibility of mid-winter
deacclimation in emerald ash borer. Biological Invasions, 14(1), 115-125.
Sodhi, N.S., D. Bickford, A.C. Diesmos, T.M. Lee, L.P. Koh, B.W. Brook, C.H. Sekercioglu,
and C.J.A. Bradshaw, 2008: Measuring the meltdown: drivers of global
amphibian extinction and decline. PLoS One, 3(2), e1636, doi:10.1371/
journal.pone.0001636.
Soja, A.J., N.M. Tchebakova, N.H.F. French, M.D. Flannigan, H.H. Shugart, B.J. Stocks,
A.I. Sukhinin, E.I. Parfenova, F.S. Chapin, and P.W. Stackhouse, 2007: Climate-
induced boreal forest change: predictions versus current observations. Global
and Planetary Change, 56(3-4), 274-296.
Sokolov, L., 2006: Effect of global warming on the timing of migration and breeding
of passerine birds in the 20
th
century. Entomological Review Supplement, 86(1),
S59-S81.

355
Terrestrial and Inland Water Systems Chapter 4
4
Soliani, C., L. Gallo, and P. Marchelli, 2012: Phylogeography of two hybridizing southern
beeches (Nothofagus spp.) with different adaptive abilities. Tree Genetics &
Genomes, 8(4), 659-673.
Sommer, J.H., H. Kreft, G. Kier, W. Jetz, J. Mutke, and W. Barthlott, 2010: Projected
impacts of climate change on regional capacities for global plant species
richness. Proceedings of the Royal Society B, 277(1692), 2271-2280.
Søndergaard, M., J.P. Jensen, and E. Jeppesen, 2003: Role of sediment and internal
loading of phosphorus in shallow lakes. Hydrobiologia, 506(1-3), 135-145.
Sovacool, B.K., 2009: Contextualizing avian mortality: a preliminary appraisal of bird
and bat fatalities from wind, fossil-fuel, and nuclear electricity. Energy Policy,
32(6), 2241-2248.
Sowerby, A., B.A. Emmett, A. Tietema, and C. Beier, 2008: Contrasting effects of
repeated summer drought on soil carbon efflux in hydric and mesic heathland
soils. Global Change Biology, 14(10), 2388-2404.
Sowerby, A., B.A. Emmett, D. Williams, C. Beier, and C.D. Evans, 2010: The response
of dissolved organic carbon (DOC) and the ecosystem carbon balance to
experimental drought in a temperate shrubland. European Journal of Soil
Science, 61(5), 697-709.
Spracklen, D.V., B. Bonn, and K.S. Carslaw, 2008: Boreal forests, aerosols and the
impacts on clouds and climate. Philosophical Transactions of the Royal Society
A, 366(1885), 4613-4626.
Stahlschmidt, Z.R., D.F. DeNardo, J.N. Holland, B.P. Kotler, and M. Kruse-Peeples,
2011: Tolerance mechanisms in North American deserts: biological and societal
approaches to climate change. Journal of Arid Environments, 75(8), 681-687.
Staver, A.C., S. Archibald, and S.A. Levin, 2011: The global extent and determinants of
savanna and forest as alternative biome states. Science, 334(6053), 230-232.
Ste-Marie, C., E.A. Nelson, A. Dabros, and M.E. Bonneau, 2011: Assisted migration:
introduction to a multifaceted concept. Forestry Chronicle, 87(6), 724-730.
Steenberg, J.W.N., P.N. Duinker, and P.G. Bush, 2011: Exploring adaptation to climate
change in the forests of central Nova Scotia, Canada. Forest Ecology and
Management, 262(12), 2316-2327.
Steffen, W., A. Persson, L. Deutsch, J. Zalasiewicz, M. Williams, K. Richardson, C.
Crumley, P. Crutzen, C. Folke, L. Gordon, M. Molina, V. Ramanathan, J. Rockstrom,
M. Scheffer, H.J. Schellnhuber, and U. Svedin, 2011: The Anthropocene: from
global change to planetary stewardship. Ambio, 40(7), 739-761.
Steffensen, J.P., K.K. Andersen, M. Bigler, H.B. Clausen, D. Dahl-Jensen, H. Fischer, K.
Goto-Azuma, M. Hansson, S.J. Johnsen, J. Jouzel, V. Masson-Delmotte, T. Popp,
S.O. Rasmussen, R. Rothlisberger, U. Ruth, B. Stauffer, M.L. Siggaard-Andersen,
A.E. Sveinbjornsdottir, A. Svensson, and J.W.C. White, 2008: High-resolution
Greenland Ice Core data show abrupt climate change happens in few years.
Science, 321(5889), 680-684.
Stern, N., 2006: The Economics of Climate Change. Cambridge University Press,
Cambridge, UK, 712 pp.
Stevens, C.J., C. Dupre, E. Dorland, C. Gaudnik, D.J.G. Gowing, A. Bleeker, M. Diekmann,
D. Alard, R. Bobbink, D. Fowler, E. Corcket, J.O. Mountford, V. Vandvik, P.A. Aarrestad,
S. Muller, and N.B. Dise, 2010: Nitrogen deposition threatens species richness
of grasslands across Europe. Environmental Pollution, 158(9), 2940-2945.
Stevens, V.M., C. Turlure, and M. Baguette, 2010: A meta-analysis of dispersal in
butterflies. Biological Reviews, 85(3), 625-642.
Stewart, I.T., 2009: Changes in snowpack and snowmelt runoff for key mountain
regions. Hydrological Processes, 23(1), 78-94.
Stewart, I.T., D.R. Cayan, and M.D. Dettinger, 2005: Changes toward earlier
streamflow timing across western North America. Journal of Climate, 18(8),
1136-1155.
Stewart, J.B., 1988: Modelling surface conductance of pine forest. Agricultural and
Forest Meteorology, 43, 19-35.
Stinson, G., W.A. Kurz, C.E. Smyth, E.T. Neilson, C.C. Dymond, J.M. Metsaranta, C.
Goisvenue, C.J. Rampley, Q. Li, T.M. White, and D. Blain, 2011: An inventory-
based analysis of Canada’s managed forest carbon dynamics, 1990 to 2008.
Global Change Biology, 17(6), 2227-2244.
Stirling, I. and A.E. Derocher, 2012: Effects of climate warming on polar bears: a
review of the evidence. Global Change Biology, 18(9), 2694-2706.
Stow, D., A. Petersen, A. Hope, R. Engstrom, and L. Coulter, 2007: Greenness trends
of Arctic tundra vegetation in the 1990s: comparison of two NDVI data sets
from NOAA AVHRR systems. International Journal of Remote Sensing, 28, 4807-
4822.
Straile, D., R. Adrian, and D.E. Schindler, 2012: Uniform temperature dependency in
the phenology of a keystone herbivore in lakes of the Northern Hemisphere.
PLoS One, 7(10), e45497, doi:10.1371/journal.pone.0045497.
Strayer, D.L. and D. Dudgeon, 2010: Freshwater biodiversity conservation: recent
progress and future challenges. Journal of the North American Benthological
Society, 29(1), 344-358.
Sturm, M., J. Schimel, G. Michaelson, J.M. Welker, S.F. Oberbauer, G.E. Liston, J.
Fahnestock, and V.E. Romanovsky, 2005: Winter biological processes could help
convert arctic tundra to shrubland. BioScience, 55(1), 17-26.
Suarez, F., D. Binkley, M.W. Kaye, and R. Stottlemyer, 1999: Expansion of forest stands
into tundra in the Noatak National Preserve, northwest Alaska. Ecoscience,
6(3), 465-470.
Suggitt, A.J., C. Stefanescu, F. Paramo, T. Oliver, B.J. Anderson, J.K. Hill, D.B. Roy, T.
Brereton, and C.D. Thomas, 2012: Habitat associations of species show consistent
but weak responses to climate. Biology Letters, 8(4), 590-593.
Sunday, J.M., A.E. Bates, and N.K. Dulvy, 2012: Thermal tolerance and the global
redistribution of animals. Nature Climate Change, 2(9), 686-690.
Suttle, K.B., M.A. Thomsen, and M.E. Power, 2007: Species interactions reverse
grassland responses to changing climate. Science, 315(5812), 640-642.
Swab, R.M., H.M. Regan, D.A. Keith, T.J. Regan, and M.K.J. Ooi, 2012: Niche models
tell half the story: spatial context and life-history traits influence species
responses to global change. Journal of Biogeography, 39(7), 1266-1277.
Syvitski, J.P.M., A.J. Kettner, I. Overeem, E.W.H. Hutton, M.T. Hannon, G.R.
Brakenridge, J. Day, C. Vorosmarty, Y. Saito, L. Giosan, and R.J. Nicholls,
2009: Sinking deltas due to human activities. Nature Geoscience, 2(10), 681-
686.
Szabo, J.K., N. Khwaja, S.T. Garnett, and S.H.M. Butchart, 2012: Global patterns and
drivers of avian extinctions at the species and subspecies level. PLoS One,
7(10), e47080, doi:10.1371/journal.pone.0047080.
Szeicz, J.M. and G.M. Macdonald, 1995: Recent white spruce dynamics at the
subarctic alpine treeline of north-western Canada. Journal of Ecology, 83(5),
873-885.
Tarnocai, C., J.G. Canadell, E.A.G. Schuur, P. Kuhry, G. Mazhitova, and S. Zimov, 2009:
Soil organic carbon pools in the northern circumpolar permafrost region. Global
Biogeochemical Cycles, 23(2), GB2023, doi:10.1029/2008GB003327.
Taylor, S., L. Kumar, N. Reid, and D.J. Kriticos, 2012: Climate change and the potential
distribution of an invasive shrub, Lantana camara L. PLoS One, 7(4), e35565.
doi:10.1371/journal.pone.0035565.
Tchebakova, N.M., E. Parfenova, and A.J. Soja, 2009: The effects of climate,
permafrost and fire on vegetation change in Siberia in a changing climate.
Environmental Research Letters, 4(4), 045013, doi:10.1088/1748-9326/4/4/
045013.
TEEB, 2009: TEEB Climate Issues Update. The Economics of Ecosystems and
Biodiversity (TEEB). Hosted by the United Nations Environment Programme
(UNEP), UNEP TEEB, Geneva, Switzerland, 32 pp.
Teixiera, E., G. Fischer, H. van Veldhuizen, R. van Dingenen, F. Dentener, G. Mills, C.
Walter, and F. Ewert, 2011: Limited potential of crop management for mitigating
surface impacts on global food supply. Atmospheric Environment, 45(15),
2569-2576.
Telwala, Y., B.W. Brook, K. Manish, and M.K. Pandit, 2013: Climate-induced
elevational range shifts and increase in plant species richness in a
Himalayan biodiversity epicentre. PLoS One, 8(2), e57103, doi:10.1371/
journal.pone.0057103.
ten Brink, P., A. Chiabai, M. Rayment, N. Braeuer, N. Peralta Bezerra, M. Kettunen,
and L. Braat, 2008: The cost of policy inaction – in monetary terms. In: The Cost
of Policy Inaction. The Case of Not Meeting the 2010 Biodiversity Target [Braat,
L.L. and P. ten Brink (eds.)]. Alterra-rapport 1718, Alterra, Wageningen University
and Research and Institute for European Environmental Policy, Cereales
Publishers, Wageningen, Netherlands, pp. 169-224.
Terrier, A., M.P. Girardin, C. Perié, P. Legendre, and Y. Bergeron, 2013: Potential
changes in forest composition could reduce impacts of climate change on
boreal wildfires. Ecological Applications, 23(1), 21-35.
Teuling, A.J., M. Hirschi, A. Ohmura, M. Wild, M. Reichstein, P. Ciais, N. Buchmann, C.
Ammann, L. Montagnani, A.D. Richardson, G. Wohlfahrt, and S.I. Seneviratne,
2009: A regional perspective on trends in continental evaporation. Geophysical
Research Letters, 36(2), L02404, doi:10.1029/2008GL036584.
Thackeray, S.J., T.H. Sparks, M. Frederiksen, S. Burthe, P.J. Bacon, J.R. Bell, M.S.
Botham, T.M. Brereton, P.W. Bright, L. Carvalho, T. Clutton-Brock, A. Dawson,
M. Edwards, I.D. Jones, J.T. Jones, D.I. Leech, D.B. Roy, W.A. Scott, M. Smith, R.J.
Smithers, I.J. Winfield, and S. Wanless, 2010: Trophic level asynchrony in rates
of phenological change for marine, freshwater and terrestrial environments.
Global Change Biology, 16(12), 3304-3313.

356
Chapter 4 Terrestrial and Inland Water Systems
4
Thaxter, C.B., A.C. Joys, R.D. Gregory, S.R. Baillie, and D.G. Noble, 2010: Hypotheses
to explain patterns of population change among breeding bird species in
England. Biological Conservation, 143(9), 2006-2019.
The Royal Society, 2008: Ground-level Ozone in the 21
st
Century: Future Trends,
Impacts and Policy Implications. Science Policy Series Report 15/08, London,
UK, 132 pp.
Thomas, C.D., A.M.A. Franco, and J.K. Hill, 2006: Range retractions and extinction in
the face of climate warming. Trends in Ecology & Evolution, 21(8), 415-416.
Thomas, C.D., P.K. Gillingham, R.B. Bradbury, D.B. Roy, B.J. Anderson, J.M. Baxter,
N.A.D. Bourn, H.Q.P. Crick, R.A. Findon, R. Fox, J.A. Hodgson, A.R. Holt, M.D.
Morecroft, N.J. O’Hanlon, T.H. Oliver, J.W. Pearce-Higgins, D.A. Procter, J.A.
Thomas, K.J. Walker, C.A. Walmsley, R.J. Wilson, and J.K. Hill, 2012: Protected
areas facilitate species’ range expansions. Proceedings of the National Academy
of Sciences of the United States of America, 109(35), 14063-14068.
Thomas, J.A., D.J. Simcox, and R.T. Clarke, 2009: Successful conservation of a
threatened Maculinea butterfly. Science, 325(5936), 80-83.
Thompson, P.L., M.C. Jacques, and R.D. Vinebrooke, 2008: Impacts of climate warming
and nitrogen deposition on alpine plankton in lake and pond habitats: an in
vitro experiment. Arctic Antarctic and Alpine Research, 40(1), 192-198.
Thonicke, K., S. Venevsky, S. Sitch, and W. Cramer, 2001: The role of fire disturbance
for global vegetation dynamics: coupling fire into a Dynamic Global Vegetation
Model. Global Ecology and Biogeography, 10, 661-667.
Thornton, P.K., J. van de Steeg, A. Notenbaert, and M. Herrero, 2009: The impacts of
climate change on livestock and livestock systems in developing countries: a
review of what we know and what we need to know. Agricultural Systems,
101(3), 113-127.
Thorup, K., A.P. Tøttrup, and C. Rahbek, 2007: Patterns of phenological changes in
migratory birds. Oecologia, 151(4), 697-703.
Throop, H.L. and S.R. Archer, 2008: Shrub (Prosopis velutina) encroachment in a
semidesert grassland: spatial–temporal changes in soil organic carbon and
nitrogen pools. Global Change Biology, 14(10), 2420-2431.
Thuiller, W., S. Lavorel, M.B. Araujo, M.T. Sykes, and I.C. Prentice, 2005: Climate
change threats to plant diversity in Europe. Proceedings of the National
Academy of Sciences of the United States of America, 102(23), 8245-8250.
Tian, H.D., L.C. Stige, B. Cazelles, K.L. Kausrud, R. Svarverud, N.C. Stenseth, and
Z.B. Zhang, 2011: Reconstruction of a 1,910-y-long locust series reveals
consistent associations with climate fluctuations in China. Proceedings of the
National Academy of Sciences of the United States of America, 108(35), 14521-
14526.
Tilman, D., C. Balzer, J. Hill, and B.L. Befort, 2011: Global food demand and the
sustainable intensification of agriculture. Proceedings of the National Academy
of Sciences of the United States of America, 108(50), 20260-20264.
Tingley, M.W., M.S. Koo, C. Moritz, A.C. Rush, and S.R. Beissinger, 2012: The push
and pull of climate change causes heterogeneous shifts in avian elevational
ranges. Global Change Biology, 18(11), 3279-3290.
Tirado, M.C., M.J. Cohen, N. Aberman, J. Meerman, and B. Thompson, 2010: Addressing
the challenges of climate change and biofuel production for food and nutrition
security. Food Research International, 43(7), 1729-1744.
Tisseuil, C., M. Vrac, G. Grenouillet, A.J. Wade, M. Gevrey, T. Oberdorff, J.B. Grodwohl,
and S. Lek, 2012: Strengthening the link between climate, hydrological and
species distribution modeling to assess the impacts of climate change on
freshwater biodiversity. Science of the Total Environment, 424, 193-201.
Tng, D.Y.P., B.P. Murphy, E. Weber, G. Sanders, G.J. Williamson, J. Kemp, and D.M.J.S.
Bowman, 2012: Humid tropical rain forest has expanded into eucalypt forest
and savanna over the last 50 years. Ecology and Evolution, 2(1), 34-45.
Tockner, K., S.E. Bunn, C. Gordon, R.J. Naiman, G.P. Quinn, and J.A. Stanford, 2008:
Floodplains: critically threatened ecosystems. In: Aquatic Ecosystems. Trends
and Global Prospects. [Polunin, N.V.C. (ed.)]. Cambridge Press, Cambridge, UK,
pp. 45-61.
Tomppo, E., T. Gschwantner, M. Lawrence, and R.E. McRoberts (eds.), 2010: National
Forest Inventories – Pathways for Common Reporting. Springer, New York, NY,
USA, 612 pp.
Traill, L.W., C.J.A. Bradshaw, S. Delean, and B.W. Brook, 2010: Wetland conservation
and sustainable use under global change: a tropical Australian case study using
magpie geese. Ecography, 33(5), 818-825.
Trathan, P.N., P.T. Fretwell, and B. Stonehouse, 2011: First recorded loss of an emperor
penguin colony in the recent period of Antarctic regional warming: implications
for other colonies. PLoS One, 6(2), e14738, doi:10.1371/journal.pone.
0014738.
Trivedi, M.R., P.M. Berry, M.D. Morecroft, and T.P. Dawson, 2008: Spatial scale affects
bioclimate model projections of climate change impacts on mountain plants.
Global Change Biology, 14(5), 1089-1103.
Tseng, W.C. and C.C. Chen, 2008: Valuing the potential economic impact of climate
change on the Taiwan trout. Ecological Economics, 65(2), 282-291.
Tsoutsos, T., N. Frantzeskaki, and V. Gekas, 2005: Environmental impacts from the
solar energy technologies. Energy Policy, 33(3), 289-296.
Tubby, K.V. and J.F. Webber, 2010: Pests and diseases threatening urban trees under
a changing climate. Forestry, 83(4), 451-459.
Turetsky, M.R., E.S. Kane, J.W. Harden, R.D. Ottmar, K.L. Manies, E. Hoy, and E.S.
Kasischke, 2011: Recent acceleration of biomass burning and carbon losses in
Alaskan forests and peatlands. Nature Geoscience, 4(1), 27-31.
Turner, W.R., B.A. Bradley, L.D. Estes, D.G. Hole, M. Oppenheimer, and D.S. Wilcove,
2010: Climate change: helping nature survive the human response. Conservation
Letters, 3(5), 304-312.
Tylianakis, J.M., R.K. Didham, J. Bascompte, and D.A. Wardle, 2008: Global change and
species interactions in terrestrial ecosystems. Ecology Letters, 11(12), 1351-1363.
Uhl, C. and J.B. Kauffman, 1990: Deforestation, fire susceptibility and potential tree
responses to fire in the eastern Amazon. Ecology, 71(2), 437-449.
UN-HABITAT, 2011: Cities and Climate Change. Global Report on Human Settlements
2011. Earthscan, London, UK and Washington DC, USA, 279 pp.
UN DESA Population Division, 2012: World Urbanization Prospects, the 2011
Revision. United Nations, Department of Economic and Social Affairs (UN
DESA), Population Division, UN Publication, New York, NY, USA, 318 pp.
Urabe, J., J. Togari, and J.J. Elser, 2003: Stoichiometric impacts of increased carbon
dioxide on a planktonic herbivore. Global Change Biology, 9(6), 818-825.
Urban, M.C., M.A. Leibold, P. Amarasekare, L. De Meester, R. Gomulkiewicz, M.E.
Hochberg, C.A. Klausmeier, N. Loeuille, C. de Mazancourt, J. Norberg, J.H. Pantel,
S.Y. Strauss, M. Vellend, and M.J. Wade, 2008: The evolutionary ecology of
metacommunities. Trends in Ecology & Evolution, 23(6), 311-317.
Urban, M.C., J.J. Tewksbury, and K.S. Sheldon, 2012: On a collision course: competition
and dispersal differences create no-analogue communities and cause extinctions
during climate change. Proceedings of the Royal Society B, 279(1735), 2072-
2080.
Uys, R.G., J.W. Bond, and T.M. Everson, 2004: The effect of different fire regimes on
plant diversity southern African grasslands. Biological Conservation, 118(4),
489-499.
Vadadi-Fulop, C., C. Sipkay, G. Meszaros, and L. Hufnagel, 2012: Climate change
and freshwater zooplankton: what does it boil down to? Aquatic Ecology,
46(4), 501-519.
Valdes, P., 2011: Built for stability. Nature Geoscience, 4(7), 414-416.
van Asch, M. and M.E. Visser, 2007: Phenology of forest caterpillars and their host
trees: the importance of synchrony. Annual Review of Entomology, 52, 37-55.
van Asch, M., P.H. Tienderen, L.J.M. Holleman, and M.E. Visser, 2007: Predicting
adaptation of phenology in response to climate change, an insect herbivore
example. Global Change Biology, 13(8), 1596-1604.
van Asch, M., L. Salis, L.J.M. Holleman, B. van Lith, and M.E. Visser, 2012: Evolutionary
response of the egg hatching date of a herbivorous insect under climate
change. Nature Climate Change, 3, 244-248.
Van Auken, O.W., 2009: Causes and consequences of woody plant encroachment into
western North American grasslands. Journal of Environmental Management,
90(10), 2931-2942.
van de Waal, D.B., A.M. Verschoor, J.M.H. Verspagen, E. van Donk, and J. Huisman, 2010:
Climate-driven changes in the ecological stoichiometry of aquatic ecosystems.
Frontiers in Ecology and the Environment, 8(3), 145-152.
van der Linde, J.A., D.L. Six, M.J. Wingfield, and J. Roux, 2011: Lasiodiplodia species
associated with dying Euphorbia ingens in South Africa. Southern Forests: a
Journal of Forest Science, 73(3-4), 165-173.
van der Molen, M.K., B.J.J.M. van den Hurk, and W. Hazeleger, 2011: A dampened land
use change climate response towards the tropics. Climate Dynamics, 37(9-10),
2035-2043.
van der Werf, G.R., J.T. Randerson, L. Giglio, G.J. Collatz, M. Mu, P.S. Kasibhatla, D.C.
Morton, R.S. DeFries, Y. Jin, and T.T. van Leeuwen, 2010: Global fire emissions
and the contribution of deforestation, savanna, forest, agricultural, and peat
fires (1997-2009). Atmospheric Chemistry and Physics, 223, 11707-11735.
Van Herk, I.G., S.T. Gower, D.R. Bronson, and M.S. Tanner, 2011: Effects of climate
warming on canopy water dynamics of a boreal black spruce plantation.
Canadian Journal of Forest Research / Revue Canadienne De Recherche
Forestiere, 41(2), 217-227.

357
Terrestrial and Inland Water Systems Chapter 4
4
van Kleunen, M., E. Weber, and M. Fischer, 2010: A meta-analysis of trait differences
between invasive and non-invasive plant species. Ecology Letters, 13(2), 235-
245.
van Mantgem, P.J., N.L. Stephenson, J.C. Byrne, L.D. Daniels, J.F. Franklin, P.Z. Fule,
M.E. Harmon, A.J. Larson, J.M. Smith, A.H. Taylor, and T.T. Veblen, 2009:
Widespread increase of tree mortality rates in the western United States.
Science, 323(5913), 521-524.
Van Minnen, J.G., B.J. Strengers, B. Eickhout, R.J. Swart, and R. Leemans, 2008:
Quantifying the effectiveness of climate change mitigation through forest
plantations and carbon sequestration with an integrated land-use model.
Carbon Balance and Management, 3(1), 3, doi:10.1186/1750-0680-3-3.
van Vliet, M.T.H., F. Ludwig, J.J.G. Zwolsman, G.P. Weedon, and P. Kabat, 2011: Global
river temperatures and sensitivity to atmospheric warming and changes in river
flow. Water Resources Research, 47(2), W02544, doi:10.1029/2010WR009198.
van Vuuren, D.P., M.G.J. den Elzen, P.L. Lucas, B. Eickhout, B.J. Strengers, B. van Ruijven,
S. Wonink, and R. van Houdt, 2007: Stabilizing greenhouse gas concentrations
at low levels: an assessment of reduction strategies and costs. Climatic Change,
81, 119-159.
van Vuuren, D.P., J. Edmonds, M. Kainuma, K. Riahi, A. Thomson, K. Hibbard, G.C.
Hurtt, T. Kram, V. Krey, J.-F. Lamarque, T. Masui, N. Nakicenovic, S.J. Smith, and
S.K. Rose, 2011: The representative concentration pathways: an overview.
Climatic Change, 109, 5-31.
van Wilgen, B.W. and D.M. Richardson, 2012: Three centuries of managing introduced
conifers in South Africa: benefits, impacts, changing perceptions and conflict
resolution. Journal of Environmental Management, 106, 56-68.
Vaughan, N.E. and T.M. Lenton, 2011: A review of climate geoengineering proposals.
Climatic Change, 109(3-4), 745-790.
Vedder, O., S. Bouwhuis, and B.C. Sheldon, 2013: Quantitative assessment of the
importance of phenotypic plasticity in adaptation to climate change in wild bird
populations. PLoS Biol, 11(7), e1001605, doi:10.1371/journal.pbio.1001605.
Veldman, J.W. and F.E. Putz, 2011: Grass-dominated vegetation, not species-diverse
natural savanna, replaces degraded tropical forests on the southern edge of
the Amazon Basin. Biological Conservation, 144(5), 1419-1429.
Vennetier, M. and C. Ripert, 2010: Climate change impact on vegetation: lessons
from an exceptionally hot and dry decade in south-eastern France. In: Climate
Change and Variability [Simard, S. (ed.)]. InTech Open, Rijeka, Croatia, pp. 225-
242.
Verburg, P., R.E. Hecky, and H. Kling, 2003: Ecological consequences of a century of
warming in Lake Tanganyika. Science, 301(5632), 505-507.
Vieira, G., J. Bockheim, M. Guglielmin, M. Balks, A.A. Abramov, J. Boelhouwers, N.
Cannone, L. Ganzert, D.A. Gilichinsky, S. Gotyachkin, J. Lopez-Martinez, I.
Meiklejohn, R. Raffi, M. Ramos, C. Schaefer, E. Serrano, F. Simas, R. Sletten, and
D. Wagner, 2010: Thermal state of permafrost and active-layer monitoring in
the Antarctic: advances during the International Polar Year 2007-2009.
Permafrost and Periglacial Processes, 21(2), 182-197.
Viglizzo, E.F., F.C. Frank, L.V. Carreno, E.G. Jobbagy, H. Pereyra, J. Clatt, D. Pincen, and
M.F. Ricard, 2011: Ecological and environmental footprint of 50 years of
agricultural expansion in Argentina. Global Change Biology, 17(2), 959-973.
Vilà-Cabrera, A., J. Martínez-Vilalta, L. Galiano, and J. Retana, 2013: Patterns of forest
decline and regeneration across Scots pine populations. Ecosystems, 16, 323-
335.
Vinukollu, R.K., R. Meynadier, J. Sheffield, and E.F. Wood, 2011: Multi-model, multi-
sensor estimates of global evapotranspiration: climatology, uncertainties and
trends. Hydrological Processes, 5, 3993-4010.
Visser, M.E. and C. Both, 2005: Shifts in phenology due to global climate change:
the need for a yardstick. Proceedings of the Royal Society of London Series B,
272(1665), 2561-2569.
Visser, M.E., L.J.M. Holleman, and S.P. Caro, 2009: Temperature has a causal effect
on avian timing of reproduction. Proceedings of the Royal Society B,
276(1665), 2323-2331.
Vitt, P., K. Havens, and O. Hoegh-Guldberg, 2009: Assisted migration: part of an
integrated conservation strategy. Trends in Ecology & Evolution, 24(9), 473-
474.
Vitt, P., K. Havens, A.T. Kramer, D. Sollenberger, and E. Yates, 2010: Assisted migration
of plants: changes in latitudes, changes in attitudes. Biological Conservation,
143(1), 18-27.
Volney, W.J.A. and R.A. Fleming, 2007: Spruce budworm (Choristoneura spp.) biotype
reactions to forest and climate characteristics. Global Change Biology, 13(8),
1630-1643.
Vongraven, D. and E. Richardson, 2011: Biodiversity – status and trends of polar
bears. In: Arctic Report Card: Update for 2011 [Richter-Menge, J., M.O. Jeffries,
and J.E. Overland (eds.)]. National Oceanic and Atmospheric Administration
(NOAA) Arctic Research Program, Washington, DC, USA, pp. 75-78, www.
arctic.noaa.gov/report11/ArcticReportCard_full_report.pdf.
Vörösmarty, C.J., P. Green, J. Salisbury, and R.B. Lammers, 2000: Global water resources:
vulnerability from climate change and population growth. Science, 289(5477),
284-288.
Vörösmarty, C.J., P.B. McIntyre, M.O. Gessner, D. Dudgeon, A. Prusevich, P. Green, S.
Glidden, S.E. Bunn, C.A. Sullivan, C.R. Liermann, and P.M. Davies, 2010: Global
threats to human water security and river biodiversity. Nature, 467, pp. 555-
561, doi:10.1038/nature09440.
Vredenburg, V.T., R.A. Knapp, T.S. Tunstall, and C.J. Briggs, 2010: Dynamics of an
emerging disease drive large-scale amphibian population extinctions. Proceedings
of the National Academy of Sciences of the United States of America, 107(21),
9689-9694.
Wagner, C. and R. Adrian, 2009: Cyanobacteria dominance: quantifying the effects
of climate change. Limnology and Oceanography, 54(6), 2460-2468.
Walker, B. and J.L. Langridge, 1997: Predicting savanna vegetation structure on the
basis of plant available moisture (PAM) and plant available nutrients (PAN): a
case study from Australia. Journal of Biogeography, 24, 813-825.
Walker, B. and D. Salt, 2006: Resilience Thinking: Sustaining Ecosystems and People
in a Changing World. Island Press, Washington, DC, USA, 174 pp.
Walker, B., C.S. Holling, S.R. Carpenter, and A. Kinzig, 2004: Resilience, adaptability
and transformability in social-ecological systems. Ecology and Society, 9(2), 5,
www.ecologyandsociety.org/vol9/iss2/art5/.
Walker, D.A., H.E. Epstein, M.K. Raynolds, P. Kuss, M.A. Kopecky, G.V. Frost, F.J.A.
Daniels, M.O. Leibman, N.G. Moskalenko, G.V. Matyshak, O.V. Khitun, A.V.
Khomutov, B.C. Forbes, U.S. Bhatt, A.N. Kade, C.M. Vonlanthen, and L. Tichy,
2012: Environment, vegetation and greenness (NDVI) along the North America
and Eurasia Arctic transects. Environmental Research Letters, 7(1), 015504,
doi:10.1088/1748-9326/7/1/015504.
Walker, M.W.C., R.D. Hollister, G.H.R. Henry, L.E. Ahlquist, J.M. Alatalo, M.S. Bret-
Harte, M.P. Calef, T.V. Callaghan, A.B. Carroll, H.E. Epstein, I.S. Jonsdottir, J.A.
Klein, B. Magnusson, U. Molau, S.F. Oberbauer, S.P. Rewa, C.H. Robinson, G.R.
Shaver, K.N. Suding, C.C. Thompson, A. Tolvanen, O. Totland, P.L. Turner, C.E.
Tweedie, P.J. Webber, and P.A. Wookey, 2006: Plant community responses to
experimental warming across the tundra biome. Proceedings of the National
Academy of Sciences of the United States of America, 103(5), 1342-1346.
Walter, J., K. Grant, C. Beierkuhnlein, J. Kreyling, M. Weber, and A. Jentsch, 2012:
Increased rainfall variability reduces biomass and forage quality of temperate
grassland largely independent of mowing frequency. Agriculture Ecosystems
& Environment, 148, 1-10.
Walters, R.J., W.U. Blanckenhorn, and D. Berger, 2012: Forecasting extinction risk of
ectotherms under climate warming: an evolutionary perspective. Functional
Ecology, 26(6), 1324-1338.
Walther, G.-R., S. Berger, and M.T. Sykes, 2005: An ecological ‘footprint’ of climate
change. Proceedings of the Royal Society B, 272(1571), 1427-1432.
Walther, G.-R., A. Roques, P.E. Hulme, M.T. Sykes, P. Pysek, I. Kuehn, M. Zobel, S.
Bacher, Z. Botta-Dukat, H. Bugmann, B. Czucz, J. Dauber, T. Hickler, V. Jarosik,
M. Kenis, S. Klotz, D. Minchin, M. Moora, W. Nentwig, J. Ott, V.E. Panov, B.
Reineking, C. Robinet, V. Semenchenko, W. Solarz, W. Thuiller, M. Vila, K. Vohland,
and J. Settele, 2009: Alien species in a warmer world: risks and opportunities.
Trends in Ecology & Evolution, 24(12), 686-693.
Wang, B., J. Huang, X. Yang, B. Zhang, and M. Liu, 2010: Estimation of biomass, net
primary production and net ecosystem production of China’s forests based on
the 1999–2003 National Forest Inventory. Scandinavian Journal of Forest
Research, 25(6), 544-553.
Wang, K., E.D. Dickinson, M. Wild, and S. Liang, 2010: Evidence for decadal variation
in global terrestrial evapotranspiration between 1982 and 2002: 2. Results.
Journal of Geophysical Research: Atmospheres, 115(D20), D20113, doi:10.1029/
2010JD013847.
Wang, S., S. Kang, L. Zhang, and F. Li, 2008: Modelling hydrological response to
different land-use and climate change scenarios in the Zamu River basin of
northwest China. Hydrological Processes, 22(14), 2502-2510.
Ward, D., 2005: Do we understand the causes of bush encroachment in African
savannas? African Journal of Range and Forage Science, 22(2), 101-105.
Wardle, P. and M.C. Coleman, 1992: Evidence for rising upper limits of four native
New Zealand forest trees. New Zealand Journal of Botany, 30(3), 303-314.

358
Chapter 4 Terrestrial and Inland Water Systems
4
Warren, R., J. Price, A. Fischlin, S.D. Santos, and G. Midgley, 2011: Increasing impacts
of climate change upon ecosystems with increasing global mean temperature
rise. Climatic Change, 106(2), 141-177.
Warren, R., J. VanDerWal, J. Price, J.A. Welbergen, I. Atkinson, J. Ramirez-Villegas, T.J.
Osborn, A. Jarvis, L.P. Shoo, S.E. Williams, and J. Lowe, 2013: Quantifying the
benefit of early climate change mitigation in avoiding biodiversity loss. Nature
Climate Change, 3(7), 678-682.
Watrin, J., A.M. Lezine, and C. Hely, 2009: Plant migration and plant communities at the
time of the “green Sahara”. Comptes Rendus Geoscience, 341(8-9), 656-670.
Wearn, O.R., D.C. Reuman, and R.M. Ewers, 2012: Extinction debt and windows of
conservation opportunity in the Brazilian Amazon. Science, 337(6091), 228-
232.
Webb, B.W. and F. Nobilis, 2007: Long-term changes in river temperature and the
influence of climatic and hydrological factors. Hydrological Sciences Journal /
Journal Des Sciences Hydrologiques, 52(1), 74-85.
Welp, L.R., J.T. Randerson, and H.P. Liu, 2007: The sensitivity of carbon fluxes to spring
warming and summer drought depends on plant functional type in boreal forest
ecosystems. Agricultural and Forest Meteorology, 147(3-4), 172-185.
Weng, E.S. and G.S. Zhou, 2006: Modeling distribution changes of vegetation in China
under future climate change. Environmental Modeling & Assessment, 11(1),
45-58.
West, J., S.H. Julius, P. Kareiva, C. Enquist, J.J. Lawler, B. Petersen, A.E. Johnson, and
M.R. Shaw, 2009: U.S. natural resources and climate change: concepts and
approaches for management adaptation. Environmental Management, 44(6),
1001-1021.
West, J.S., J.A. Townsend, M. Stevens, and B.D.L. Fitt, 2012: Comparative biology of
different plant pathogens to estimate effects of climate change on crop
diseases in Europe. European Journal of Plant Pathology, 133(1), 315-331.
Westerling, A., H. Hidalgo, D. Cayan, and T. Swetnam, 2006: Warming and earlier spring
increase western U.S. forest wildfire activity. Science, 313(5887), 940-943.
Westley, F., P. Olsson, C. Folke, T. Homer-Dixon, H. Vredenburg, D. Loorbach, J. Thompson,
M. Nilsson, E. Lambin, J. Sendzimir, B. Banerjee, V. Galaz, and S. van der Leeuw,
2011: Tipping toward sustainability: emerging pathways of transformation.
Ambio, 40(7), 762-780.
Weyhenmeyer, G.A., E. Jeppesen, R. Adrian, L. Arvola, T. Blenckner, T. Jankowski, E.
Jennings, P. Noges, T. Noges, and D. Straile, 2007: Nitrate-depleted conditions on
the increase in shallow northern European lakes. Limnology and Oceanography,
52(4), 1346-1353.
Weyhenmeyer, G.A., D.M. Livingstone, M. Meili, O. Jensen, B. Benson, and J.J.
Magnuson, 2011: Large geographical differences in the sensitivity of ice-
covered lakes and rivers in the Northern Hemisphere to temperature changes.
Global Change Biology, 17(1), 268-275.
White, C.R., J.A. Green, G.R. Martin, P.J. Butler, and D. Gremillet, 2013: Energetic
constraints may limit the capacity of visually guided predators to respond to
Arctic warming. Journal of Zoology, 289(2), 119-126.
White, M.A., K.M. de Beurs, K. Didan, D.W. Inouye, A.D. Richardson, O.P. Jensen, J.
O’Keefe, G. Zhang, R.R. Nemani, W.J.D. van Leeuwen, J.F. Brown, A. de Wit, M.
Schaepman, X.M. Lin, M. Dettinger, A.S. Bailey, J. Kimball, M.D. Schwartz, D.D.
Baldocchi, J.T. Lee, and W.K. Lauenroth, 2009: Intercomparison, interpretation,
and assessment of spring phenology in North America estimated from remote
sensing for 1982-2006. Global Change Biology, 15(10), 2335-2359.
Wickham, J.D., T.G. Wade, and K.H. Riitters, 2012: Empirical analysis of the influence
of forest extent on annual and seasonal surface temperatures for the
continental United States. Global Ecology and Biogeography, 22(5), 620-629.
Wiedner, C., J. Rucker, R. Bruggemann, and B. Nixdorf, 2007: Climate change affects
timing and size of populations of an invasive cyanobacterium in temperate
regions. Oecologia, 152(3), 473-484.
Wiegand, K., D. Ward, and D. Saltz, 2005: Multi-scale patterns and bush encroachment
in an arid savanna with a shallow soil layer. Journal of Vegetation Science,
16(3), 311-320.
Wiens, J.A., N.E. Seavy, and D. Jongsomjit, 2011: Protected areas in climate space:
what will the future bring? Biological Conservation, 144(8), 2119-2125.
Wigley, B.J., W.J. Bond, and M.T. Hoffman, 2009: Bush encroachment under three
contrasting land-use practices in a mesic South African savanna. African Journal
of Ecology, 47(Suppl s1), 62-70.
Wild, M., J. Grieser, and C. Schär, 2008; Combined surface solar brightening and
increasing greenhouse effect support recent intensification of the global land-
based hydrological circle. Geophysical Research Letters, 35(17), L17706,
doi:10.1029/2008GL034842.
Wiley, M.J., D.W. Hyndman, B.C. Pijanowski, A.D. Kendall, C. Riseng, E.S. Rutherford,
S.T. Cheng, M.L. Carlson, J.A. Tyler, R.J. Stevenson, P.J. Steen, P.L. Richards, P.W.
Seelbach, J.M. Koches, and R.R. Rediske, 2010: A multi-modeling approach to
evaluating climate and land use change impacts in a Great Lakes River Basin.
Hydrobiologia, 657(1), 243-262.
Wilhelm, S. and R. Adrian, 2008: Impact of summer warming on the thermal
characteristics of a polymictic lake and consequences for oxygen, nutrients and
phytoplankton. Freshwater Biology, 53(2), 226-237.
Wilkinson, S. and W.J. Davies, 2010: Drought, ozone, ABA and ethylene: new insights
from cell to plant to community. Plant Cell and Environment, 33(4), 510-525.
Williams, A.L., K.E. Wills, J.K. Janes, J.K.V. Schoor, P.C.D. Newton, and M.J. Hovenden,
2007: Warming and free-air CO
2
enrichment alter demographics in four co-
occurring grassland species. New Phytologist, 176(2), 365-374.
Williams, A.P., C.D. Allen, C.I. Millar, T.W. Swetnam, J. Michaelsen, C.J. Still, and S.W.
Leavitt, 2010: Forest responses to increasing aridity and warmth in the
southwestern United States. Proceedings of the National Academy of Sciences
of the United States of America, 107(50), 21289-21294.
Williams, A.P., C.D. Allen, A.K. Macalady, D. Griffin, C.A. Woodhouse, D.M. Meko, T.W.
Swetnam, S.A. Rauscher, R. Seager, H.D. Grissino-Mayer, J.S. Dean, E.R. Cook,
C. Gangodagamage, M. Cai, and N.G. McDowell, 2013: Temperature as a potent
driver of regional forest drought stress and tree mortality. Nature Climate
Change, 3, 292-297.
Williams, J.W. and S.T. Jackson, 2007: Novel climates, no-analog communities, and
ecological surprises. Frontiers in Ecology and the Environment, 5(9), 475-482.
Williams, J.W., S.T. Jackson, and J. E. Kutzbach, 2007: Projected distributions of novel
and disappearing climates by 2100 AD. Proceedings of the National Academy
of Sciences of the United States of America, 104(14), 5738-5742.
Williams, J.W., B. Shuman, and P.J. Bartlein, 2009: Rapid responses of the prairie-
forest ecotone to early Holocene aridity in mid-continental North America.
Global and Planetary Change, 66(3-4), 195-207.
Williams, J.W., B. Shuman, P.J. Bartlein, N.S. Diffenbaugh, and T. Webb, 2010: Rapid,
time-transgressive, and variable responses to early Holocene midcontinental
drying in North America. Geology, 38(2), 135-138.
Williams, J.W., J.L. Blois, and B.N. Shuman, 2011: Extrinsic and intrinsic forcing of
abrupt ecological change: case studies from the late Quaternary. Journal of
Ecology, 99(3), 664-677.
Willis, C.G., B.R. Ruhfel, R.B. Primack, A.J. Miller-Rushing, J.B. Losos, and C.C. Davis,
2010: Favorable climate change response explains non-native species’ success
in Thoreau’s Woods. PLoS One, 5(1), e8878, doi:10.1371/journal.pone.0008878.
Willis, C.K.R., R.M.R. Barclay, J.G. Boyles, R.M. Brigham, V. Brack Jr, D.L. Waldien, and
J. Reichard, 2010: Bats are not birds and other problems with Sovacool’s (2009)
analysis of animal fatalities due to electricity generation. Energy Policy, 38,
2067-2069.
Willis, K.J. and S.A. Bhagwat, 2009: Biodiversity and climate change. Science,
326(5954), 806-807.
Willis, K.J. and G.M. MacDonald, 2011: Long-term ecological records and their relevance
to climate change predictions for a warmer world. Annual Review of Ecology,
Evolution, and Systematics, 42, 267-287.
Willis, K.J., K.D. Bennett, S.A. Bhagwat, and H.J.B. Birks, 2010: 4 °C and beyond: what
did this mean for biodiversity in the past? Systematics and Biodiversity, 8(1),
3-9.
Willmer, P., 2012: Ecology: pollinator-plant synchrony tested by climate change.
Current Biology, 22(4), R131-R132.
Wilson, R., R. D’Arrigo, B. Buckley, U. Büntgen, J. Esper, D. Frank, B. Luckman, S.
Payette, R. Vose, and D. Youngblut, 2007: A matter of divergence: tracking recent
warming at hemispheric scales using tree ring data. Journal of Geophysical
Research: Atmospheres, 112(D17), D17103, doi:10.1029/2006JD008318.
Winder, M. and D.E. Schindler, 2004: Climatic effects on the phenology of lake
processes. Global Change Biology, 10(11), 1844-1856.
Winder, M. and U. Sommer, 2012: Phytoplankton response to a changing climate.
Hydrobiologia, 698(1), 5-16.
Winder, M., J.E. Reuter, and S.G. Schladow, 2009: Lake warming favours small-sized
planktonic diatom species. Proceedings of the Royal Society B, 276(1656),
427-435.
Winder, M., A.D. Jassby, and R. Mac Nally, 2011: Synergies between climate
anomalies and hydrological modifications facilitate estuarine biotic invasions.
Ecology Letters, 14(8), 749-757.
Winder, R., E.A. Nelson, and T. Beardmore, 2011: Ecological implications for assisted
migration in Canadian forests. Forestry Chronicle, 87(6), 731-744.

359
Terrestrial and Inland Water Systems Chapter 4
4
Wing, S.L. and E.D. Currano, 2013: Plant response to a global greenhouse event 56
million years ago. American Journal of Botany, 100(7), 1234-1254.
Wing, S.L., G.J. Harrington, F.A. Smith, J.I. Bloch, D.M. Boyer, and K.H. Freeman, 2005:
Transient floral change and rapid global warming at the Paleocene-Eocene
boundary. Science, 310(5750), 993-996.
Winter, M., O. Schweiger, S. Klotz, W. Nentwig, P. Andriopoulos, M. Arianoutsou, C.
Basnou, P. Delipetrou, V. Didziulis, M. Hejda, P.E. Hulme, P.W. Lambdon, J. Pergl,
P. Pyšek, D.B. Roy, and I. Kühn, 2009: Plant extinctions and introductions lead
to phylogenetic and taxonomic homogenization of the European flora.
Proceedings of the National Academy of Sciences of the United States of
America, 106(51), 21721-21725.
Wise, M., K. Calvin, A. Thomson, L. Clarke, B. Bond-Lamberty, R. Sands, S.J. Smith, A.
Janetos, and J. Edmonds, 2009: Implications of limiting CO
2
concentrations for
land use and energy. Science, 324(5931), 1183-1186.
Witt, G.B., R.A. Harrington, and M.J. Page, 2009: Is ‘vegetation thickening’ occurring
in Queensland’s mulga lands – a 50-year aerial photographic analysis.
Australian Journal of Botany, 57(7), 572-582.
Witte, J.C., A.R. Douglass, A. da Silva, O. Torres, R. Levy, and B.N. Duncan, 2011: NASA
A-Train and Terra observations of the 2010 Russian wildfires. Atmospheric
Chemistry and Physics, 11(17), 9287-9301.
Wittig, V.E., E.A. Ainsworth, and S.P. Long, 2007: To what extent do current and
projected increases in surface ozone affect photosynthesis and stomatal
conductance of trees? A meta-analytic review of the last 3 decades of experiments.
Plant, Cell & Environment, 30(9), 1150-1162.
Wittig, V.E., E.A. Ainsworth, S.L. Naidu, D.F. Karnosky, and S.P. Long, 2009: Quantifying
the impact of current and future tropospheric ozone on tree biomass, growth
physiology and biochemistry. Global Change Biology, 15(2), 396-424.
Woillez, M.N., M. Kageyama, G. Krinner, N. de Noblet-Ducoudre, N. Viovy, and M.
Mancip, 2011: Impact of CO
2
and climate on the Last Glacial Maximum
vegetation: results from the ORCHIDEE/IPSL models. Climate of the Past, 7(2),
557-577.
Wolken, J.M., T.N. Hollingsworth, T.S. Rupp, F.S. Chapin, S.F. Trainor, T.M. Barrett, P.F.
Sullivan, A.D. McGuire, E.S. Euskirchen, P.E. Hennon, E.A. Beever, J.S. Conn, L.K.
Crone, D.V. D’Amore, N. Fresco, T.A. Hanley, K. Kielland, J.J. Kruse, T. Patterson,
E.A.G. Schuur, D.L. Verbyla, and J. Yarie, 2011: Evidence and implications of
recent and projected climate change in Alaska’s forest ecosystems. Ecosphere,
2(11), 124, doi:10.1890/ES11-00288.1.
Wolkovich, E.M., B.I. Cook, J.M. Allen, T.M. Crimmins, J.L. Betancourt, S.E. Travers, S.
Pau, J. Regetz, T.J. Davies, N.J.B. Kraft, T.R. Ault, K. Bolmgren, S.J. Mazer, G.J.
McCabe, B.J. McGill, C. Parmesan, N. Salamin, M.D. Schwartz, and E.E. Cleland,
2012: Warming experiments underpredict plant phenological responses to
climate change. Nature, 485, 494-497.
Wood, T.E., M.A. Cavaleri, and S.C. Reed, 2012: Tropical forest carbon balance in a
warmer world: a critical review spanning microbial- to ecosystem-scale
processes. Biological Reviews, 87(4), 912-927.
Woodburne, M.O., G.F. Gunnell, and R.K. Stucky, 2009: Climate directly influences
Eocene mammal faunal dynamics in North America. Proceedings of the
National Academy of Sciences of the United States of America, 106(32), 13399-
13403.
Wookey, P.A., R. Aerts, R.D. Bardgett, F. Baptist, K.A. Brathen, J.H.C. Cornelissen, L.
Gough, I.P. Hartley, D.W. Hopkins, S. Lavorel, and G.R. Shaver, 2009: Ecosystem
feedbacks and cascade processes: understanding their role in the responses of
arctic and alpine ecosystems to environmental change. Global Change Biology,
15(5), 1153-1172.
Worrall, J.J., G.E. Rehfeldt, A. Hamann, E.H. Hogg, S.B. Marchetti, M. Michaelian, and
L.K. Gray, 2013: Recent declines of Populus tremuloides in North America linked
to climate. Forest Ecology and Management, 299, 35-51.
Wu, C.Y. and J.M. Chen, 2013: Diverse responses of vegetation production to
interannual summer drought in North America. International Journal of Applied
Earth Observation and Geoinformation, 21, 1-6.
Wu, X.B. and S.R. Archer, 2005: Scale-dependent influence of topography-based
hydrologic features on patterns of woody plant encroachment in savanna
landscapes. Landscape Ecology, 20(6), 733-742.
Wu, Z., H. Zhang, C.M. Krause, and N.S. Cobb, 2010: Climate change and human
activities: a case study in Xinjiang, China. Climatic Change, 99(3-4), 457-
472.
Xenopoulos, M.A., D.M. Lodge, J. Alcamo, M. Marker, K. Schulze, and D.P. Van Vuuren,
2005: Scenarios of freshwater fish extinctions from climate change and water
withdrawal. Global Change Biology, 11(10), 1557-1564.
Xu, L., R.B. Myneni, F.S. Chapin III, T.V. Callaghan, J.E. Pinzon, C.J. Tucker, Z. Zhu, J. Bi,
P. Ciais, H. Tømmervik, E.S. Euskirchen, B.C. Forbes, S.L. Piao, B.T. Anderson, S.
Ganguly, R.R. Nemani, S.J. Goetz, P.S.A. Beck, A.G. Bunn, C. Cao, and J.C. Stroeve,
2013: Temperature and vegetation seasonality diminishment over northern
lands. Nature Climate Change, 3, 581-586.
Yasuda, M., H. Daimaru, and S. Okitsu, 2007: Detection of alpine moor vegetation
change by comparison of orthonized aerophotographs at different times.
Geographical Review of Japan, 80, 842-856.
Yi, S.H., M.K. Woo, and M.A. Arain, 2007: Impacts of peat and vegetation on
permafrost degradation under climate warming. Geophysical Research Letters,
34(16), L16504, doi:10.1029/2007GL030550.
Yoshikawa, S. and K. Sanga-Ngoie, 2011: Deforestation dynamics in Mato Grosso in
the southern Brazilian Amazon using GIS and NOAA/AVHRR data. International
Journal of Remote Sensing, 32(2), 523-544.
Yvon-Durocher, G., J.M. Montoya, M. Trimmer, and G. Woodward, 2011: Warming
alters the size spectrum and shifts the distribution of biomass in freshwater
ecosystems. Global Change Biology, 17(4), 1681-1694.
Zarnetske, P.L., D.K. Skelly, and M.C. Urban, 2012: Biotic multipliers of climate
change. Science, 336(6088), 1516-1518.
Zavaleta, E.S., M.R. Shaw, N.R. Chiariello, B.D. Thomas, E.E. Cleland, C.B. Field, and
H.A. Mooney, 2003: Grassland responses to three years of elevated temperature,
CO
2
, precipitation, and N deposition. Ecological Monographs, 73(4), 585-604.
Zelazowski, P., Y. Malhi, C. Huntingford, S. Sitch, and J.B. Fisher, 2011: Changes in
the potential distribution of humid tropical forests on a warmer planet.
Philosophical Transactions of the Royal Society A, 369(1934), 137-160.
Zeng, Z., S. Piao, X. Lin, G. Yin, S. Peng, P. Ciais, and R.B. Myneni, 2012: Global
evapotranspiration over the past three decades: estimation based on the water
balance equation combined with empirical models. Environmental Research
Letters, 7(1), 014026, doi:10.1088/1748-9326/7/1/014026.
Zerebecki, R.A. and C.J.B. Sorte, 2011: Temperature tolerance and stress proteins as
mechanisms of invasive species success. PLoS One, 6(4), e14806, doi:10.1371/
journal.pone.0014806.
Zhang, H., Y. Li, and X. Gao, 2009: Potential impacts of land-use on climate variability
and extremes. Advances in Atmospheric Sciences, 26(5), 840-854.
Zhang, Y.Y., M. Fischer, V. Colot, and O. Bossdorf, 2013: Epigenetic variation creates
potential for evolution of plant phenotypic plasticity. New Phytologist, 197(1),
314-322.
Zhao, M. and S.W. Running, 2010: Drought-induced reduction in global terrestrial
net primary production from 2000 through 2009. Science, 329(5994), 940-
943.
Zhou, G., C. Peng, Y. Li, S. Liu, Q. Zhang, X. Tang, J. Liu, J. Yan, D. Zhang, and G. Chu,
2013: A climate change-induced threat to the ecological resilience of a
subtropical monsoon evergreen broad-leaved forest in Southern China. Global
Change Biology, 19, 1197-1210.
Zhou, Y.P., K.M. Xu, Y.C. Sud, and A.K. Betts, 2011: Recent trends of the tropical
hydrological cycle inferred from Global Precipitation Climatology Project and
International Satellite Cloud Climatology Project data. Journal of Geophysical
Research: Atmospheres, 116(D9), D09101, doi:10.1029/2010JD015197.
Zhu, K., C.W. Woodall, and J.S. Clark, 2012: Failure to migrate: lack of tree range
expansion in response to climate change. Global Change Biology, 18(3), 1042-
1052.
Zhu, Z.L., Z.Q. Xiong, and G.X. Xing, 2005: Impacts of population growth and
economic development on the nitrogen cycle in Asia. Science in China Series
C: Life Sciences, 48, 729-737.
Zimmermann, N.E., N.G. Yoccoz, T.C. Edwards, E.S. Meier, W. Thuiller, A. Guisan, D.R.
Schmatz, and P.B. Pearman, 2009: Climatic extremes improve predictions of
spatial patterns of tree species. Proceedings of the National Academy of
Sciences of the United States of America, 106, 19723-19728.
Zimov, N.S., S.A. Zimov, A.E. Zimova, G.M. Zimova, V.I. Chuprynin, and F.S. Chapin,
2009: Carbon storage in permafrost and soils of the mammoth tundra-steppe
biome: role in the global carbon budget. Geophysical Research Letters, 36(2),
L02502, doi:10.1029/2008GL036332.
Zurell, D., V. Grimm, E. Rossmanith, N. Zbinden, N.E. Zimmermann, and B. Schroder,
2012: Uncertainty in predictions of range dynamics: black grouse climbing the
Swiss Alps. Ecography, 35(7), 590-603.
Zyryanova, O.A., V. T. Yaborov, T. I. Tchikhacheva, T. Koike, K. Makoto, Y. Matsuura, F.
Satoh, and V. I. Zyryanov, 2007: The structure and biodiversity after fire
disturbance in Larix gmelinii (Rupr.) Rupr. forests, northeasten Asia. Eurasian
Journal of Forest Research, 10(1), 19-29.
