
1567
28
Polar Regions
Coordinating Lead Authors:
Joan Nymand Larsen (Iceland), Oleg A. Anisimov (Russian Federation)
Lead Authors:
Andrew Constable (Australia), Anne B. Hollowed (USA), Nancy Maynard (USA), Pål Prestrud
(Norway), Terry D. Prowse (Canada), John M.R. Stone (Canada)
Contributing Authors:
Terry V. Callaghan (UK), Mark Carey (USA), Peter Convey (UK), Andrew Derocher (Canada),
Bruce C. Forbes (Finland), Peter T. Fretwell (UK), Solveig Glomsrød (Norway), Dominic Hodgson
(UK), Eileen Hofmann (USA), Grete K. Hovelsrud (Norway), Gita L. Ljubicic (Canada),
Harald Loeng (Norway), Eugene Murphy (UK), Steve Nicol (Australia), Anders Oskal (Norway),
James D. Reist (Canada), Phil Trathan (UK), Barbara Weinecke (Australia), Fred Wrona
(Canada)
Review Editors:
Maria Ananicheva (Russian Federation), F. Stuart Chapin III (USA)
Volunteer Chapter Scientist:
Vasiliy Kokorev (Russian Federation)
This chapter should be cited as:
Larsen
, J.N., O.A. Anisimov, A. Constable, A.B. Hollowed, N. Maynard, P. Prestrud, T.D. Prowse, and J.M.R. Stone, 2014:
Polar regions. In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution
of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Barros, V.R.,
C.B. Field, D.J. Dokken, M.D. Mastrandrea, K.J. Mach, T.E. Bilir, M. Chatterjee, K.L. Ebi, Y.O. Estrada, R.C. Genova,
B. Girma, E.S. Kissel, A.N. Levy, S. MacCracken, P.R. Mastrandrea, and L.L. White (eds.)]. Cambridge University Press,
Cambridge, United Kingdom and New York, NY, USA, pp. 1567-1612.

28
1568
Executive Summary ......................................................................................................................................................... 1570
28.1. Introduction .......................................................................................................................................................... 1572
28.2. Observed Changes and Vulnerability under Multiple Stressors ........................................................................... 1572
28.2.1. Hydrology and Freshwater Ecosystems ........................................................................................................................................... 1572
28.2.1.1. Arctic ............................................................................................................................................................................... 1572
28.2.1.2. Antarctic .......................................................................................................................................................................... 1573
28.2.2. Oceanography and Marine Ecosystems .......................................................................................................................................... 1574
28.2.2.1. Arctic ............................................................................................................................................................................... 1574
28.2.2.2. Antarctica ........................................................................................................................................................................ 1576
28.2.3. Terrestrial Ecosystems ..................................................................................................................................................................... 1577
28.2.3.1. Arctic ............................................................................................................................................................................... 1577
28.2.3.2. Antarctica ........................................................................................................................................................................ 1581
28.2.4. Health and Well-being of Arctic Residents ...................................................................................................................................... 1581
28.2.4.1. Direct Impacts of a Changing Climate on the Health of Arctic Residents ........................................................................ 1581
28.2.4.2. Indirect Impacts of Climate Change on the Health of Arctic Residents ............................................................................ 1582
28.2.5. Indigenous Peoples and Traditional Knowledge .............................................................................................................................. 1583
28.2.6. Economic Sectors ............................................................................................................................................................................ 1584
28.2.6.1. Arctic ............................................................................................................................................................................... 1584
28.2.6.2. Antarctica and the Southern Ocean ................................................................................................................................. 1585
28.3. Key Projected Impacts and Vulnerabilities ........................................................................................................... 1586
28.3.1. Hydrology and Freshwater Ecosystems ........................................................................................................................................... 1586
28.3.1.1. Arctic ............................................................................................................................................................................... 1586
28.3.1.2. Antarctica ........................................................................................................................................................................ 1586
28.3.2. Oceanography and Marine Ecosystems .......................................................................................................................................... 1587
28.3.2.1. Ocean Acidification in the Arctic and Antarctic ................................................................................................................ 1587
28.3.2.2. Arctic ............................................................................................................................................................................... 1587
28.3.2.3. Antarctica and the Southern Ocean ................................................................................................................................. 1589
28.3.3. Terrestrial Environment and Related Ecosystems ............................................................................................................................ 1589
28.3.3.1. Arctic ............................................................................................................................................................................... 1589
28.3.3.2. Antarctica ........................................................................................................................................................................ 1590
28.3.4. Economic Sectors ............................................................................................................................................................................ 1590
28.3.4.1. Fisheries .......................................................................................................................................................................... 1590
28.3.4.2. Forestry and Farming ....................................................................................................................................................... 1591
28.3.4.3. Infrastructure, Transportation, and Terrestrial Resources ................................................................................................. 1591
Table of Contents

1569
Polar Regions Chapter 28
28
28.4. Human Adaptation ................................................................................................................................................ 1593
28.5. Research and Data Gaps ....................................................................................................................................... 1595
References ....................................................................................................................................................................... 1596
Frequently Asked Questions
28.1: What will be the net socioeconomic impacts of change in the polar regions? ............................................................................... 1595
28.2: Why are changes in sea ice so important to the polar regions? ...................................................................................................... 1596

1570
Chapter 28 Polar Regions
28
Executive Summary
Additional and stronger scientific evidence has accumulated since the AR4 that reinforces key findings made in the Fourth Assessment Report
(AR4).
The impacts of climate change, and the adaptations to it, exhibit strong spatial heterogeneity in the polar regions because of the
high diversity of social systems, biophysical regions, and associated drivers of change (high confidence). {28.2.2} For example, the
tree line has moved northward and upward in many, but not all, Arctic areas (high confidence) and significant increases in tall shrubs and
grasses have been observed in many places (very high confidence). {28.2.3.1.2}
Some marine species will shift their ranges in response to changing ocean and sea ice conditions in the polar regions (medium
confidence). The response rate and the spatial extent of the shifts will differ by species based on their vulnerability to change and their life
history. {28.2.2, 28.3.2} Loss of sea ice in summer and increased ocean temperatures are expected to impact secondary pelagic production in
some regions of the Arctic Ocean, with associated changes in the energy pathways within the marine ecosystem (medium confidence). These
changes are expected to alter the species composition of zooplankton in some regions, with associated impacts on some fish and shellfish
populations (medium confidence). {28.2.2.1} Also, changes in sea ice and the physical environment to the west of the Antarctic Peninsula are
altering phytoplankton stocks and productivity, and krill (high confidence). {28.2.2.2}
Climate change is impacting terrestrial and freshwater ecosystems in some areas of Antarctica and the Arctic. This is due to
ecological effects resulting from reductions in the duration and extent of ice and snow cover and enhanced permafrost thaw (very high
confidence), and through changes in the precipitation-evaporation balance (medium confidence). {28.2.1, 28.2.3}
The primary concern for polar bears over the foreseeable future is the recent and projected loss of annual sea ice cover, decreased
ice duration, and decreased ice thickness (high confidence).
Of the two subpopulations where data are adequate for assessing abundance
effects, it is very likely that the recorded population declines are caused by reductions in sea ice extent. {28.2.2.1.2, 28.3.2.2.2}
Rising temperatures, leading to the further thawing of permafrost, and changing precipitation patterns have the potential to
affect infrastructure and related services in the Arctic (high confidence). {28.3.4.3} Particular concerns are associated with damage to
residential buildings resulting from thawing permafrost, including Arctic cities; small, rural settlements; and storage facilities for hazardous
materials. {28.2.4-5}
In addition, there is new scientific evidence that has emerged since the AR4.
The physical, biological, and socioeconomic impacts of climate change in the Arctic have to be seen in the context of often
interconnected factors that include not only environmental changes caused by drivers other than climate change but also
demography, culture, and economic development.
Climate change has compounded some of the existing vulnerabilities caused by these
other factors (high confidence). {28.2.4-5, 28.4} For example, food security for many Indigenous and rural residents in the Arctic is being
impacted by climate change, and in combination with globalization and resource development food insecurity is projected to increase in the
future (high confidence). {28.2.4}
The rapid rate at which climate is changing in the polar regions will impact natural and social systems (high confidence) and may
exceed the rate at which some of their components can successfully adapt (low to medium confidence). {28.2.4, 28.4}
The decline
of Arctic sea ice in summer is occurring at a rate that exceeds most of the earlier generation model projections (high confidence), and evidence
of similarly rapid rates of change is emerging in some regions of Antarctica. {WGI AR5 Chapters 4, 5, 9} In the future, trends in polar regions of
populations of marine mammals, fish, and birds will be a complex response to multiple stressors and indirect effects (high confidence). {28.3.2}
Already, accelerated rates of change in permafrost thaw, loss of coastal sea ice, sea level rise, and increased weather intensity are forcing
relocation of some Indigenous communities in Alaska (high confidence). {28.2.4.2, 28.2.5, 28.3.4}

1571
28
Polar Regions Chapter 28
Shifts in the timing and magnitude of seasonal biomass production could disrupt matched phenologies in the food webs, leading
to decreased survival of dependent species (medium confidence).
If the timing of primary and secondary production is no longer
matched to the timing of spawning or egg release, survival could be impacted, with cascading implications to higher trophic levels. This impact
would be exacerbated if shifts in timing occur rapidly (medium confidence). {28.2.2, 28.3.2} Climate change will increase the vulnerability of
terrestrial ecosystems to invasions by non-indigenous species, the majority likely to arrive through direct human assistance (high confidence).
Ocean acidification has the potential to inhibit embryo development and shell formation of some zooplankton and krill in the
polar regions, with potentially far-reaching consequences to food webs in these regions (medium confidence). Embryos of Antarctic
krill have been shown to be vulnerable to increased concentrations of carbon dioxide (CO
2
) in the water (high confidence). As well, there is
increasing evidence that pelagic molluscs (pteropods) are vulnerable to ocean acidification (medium confidence). {28.2.2, 28.3.2}
There is increased evidence that climate change will have large effects on Arctic communities, especially where narrowly based
economies leave a smaller range of adaptive choices. {28.2.6.1, 28.4} Some commercial activities will become more profitable while
others will face decline. Increased economic opportunities are expected with increased navigability in the Arctic Ocean and the expansion of
some land- and freshwater-based transportation networks. {28.2.6.1.3, 28.3.4.3} The informal, subsistence-based economy will be impacted
(high confidence). There is high confidence that changing sea ice conditions may result in more difficult access for hunting marine mammals.
{28.2.6.1.6} Although Arctic residents have a history of adapting to change, the complex interlinkages among societal, economic, and political
factors and climatic stresses represent unprecedented challenges for northern communities, particularly if the rate of change will be faster than
the social systems can adapt (high confidence). {28.2.5, 28.4}
Impacts on the health and well-being of Arctic residents from climate change are significant and projected to increase—especially
for many Indigenous peoples (high confidence). {28.2.4} These impacts are expected to vary among the diverse settlements, which range
from small, remote, predominantly Indigenous communities to large cities and industrial settlements (high confidence), especially those in
highly vulnerable locations along ocean and river shorelines. {28.2.4}
1572
Chapter 28 Polar Regions
28
28.1. Introduction
Several recent climate impact assessments on polar regions have been
undertaken, including the synthesis report on Snow, Water, Ice and
P
ermafrost in the Arctic (AMAP, 2011a), the State of the Arctic Coast
2010 (2011) reports, the Antarctic Climate and the Environment (Turner
et al., 2009, 2013), Arctic Resilience Interim Report 2013 (2013), and
the findings of the International Polar Year (IPY; Krupnick et al., 2011).
These reports draw a consistent pattern of climate-driven environmental,
societal, and economic changes in the polar regions in recent decades.
In this chapter, we use the scientific literature, including these reports,
to consolidate the assessment of the impacts of climate change on polar
regions from 2007, advance new scientific evidence of impacts, and
identify key gaps in knowledge on current and future impacts. Previous
IPCC reports define the Arctic as the area within the Arctic Circle (66ºN),
and the Antarctic as the continent with surrounding Southern Ocean
south of the polar front, which is generally close to 58ºS (IPCC, 2007).
For the purpose of this report we use the conventional IPCC definitions
as a basis, while incorporating a degree of flexibility when describing
the polar regions in relation to particular subjects.
Changes in the physical and chemical environments of the polar regions
are detailed in the WGI contribution to the AR5. There is evidence that
Arctic land surface temperatures have warmed substantially since the
mid-20th century, and the futurerate of warming is expected to exceed
the global rate. Sea ice extent at the summer minimum has decreased
significantly in recent decades, and the Arctic Ocean is projected to
become nearly ice free in summer within this century. The duration of
snow cover extent and snow depth are decreasing in North America while
i
ncreasing in Eurasia. Since the late 1970s, permafrost temperatures
have increased between 0.5°C and 2°C. In the Southern Hemisphere,
the strongest rates of atmospheric warming are occurring in the western
Antarctic Peninsula (WAP, between 0.2°C and 0.3°C per decade) and
the islands of the Scotia Arc, where there have also been increases in
oceanic temperatures and large regional decreases in winter sea ice
extent and duration. Warming, although less than WAP, has also occurred
in the continental margins near the Bellingshausen Sea, Prydz Bay, and
the Ross Sea, with areas of cooling in between. Land regions have
experienced glacial recession and changes in the ice and permafrost
habitats in the coastal margins. The Southern Ocean continues to warm,
with increased freshening at the surface due to precipitation leading to
increased stratification. In both polar regions, as a result of acidification,
surface waters will become seasonally corrosive to aragonite within
decades, with some regions being affected sooner than others (see
Box CC-OA; WGI AR5 Chapter 6). Observations and models indicate that
the carbon cycle of the Arctic and Southern Oceans will be impacted by
climate change and increased carbon dioxide (CO
2
).
28.2. Observed Changes and Vulnerability
under Multiple Stressors
28.2.1. Hydrology and Freshwater Ecosystems
28.2.1.1. Arctic
Arctic rivers and lakes continue to show pronounced changes to their
hydrology and ecology. Previously noted increases in Eurasian Arctic
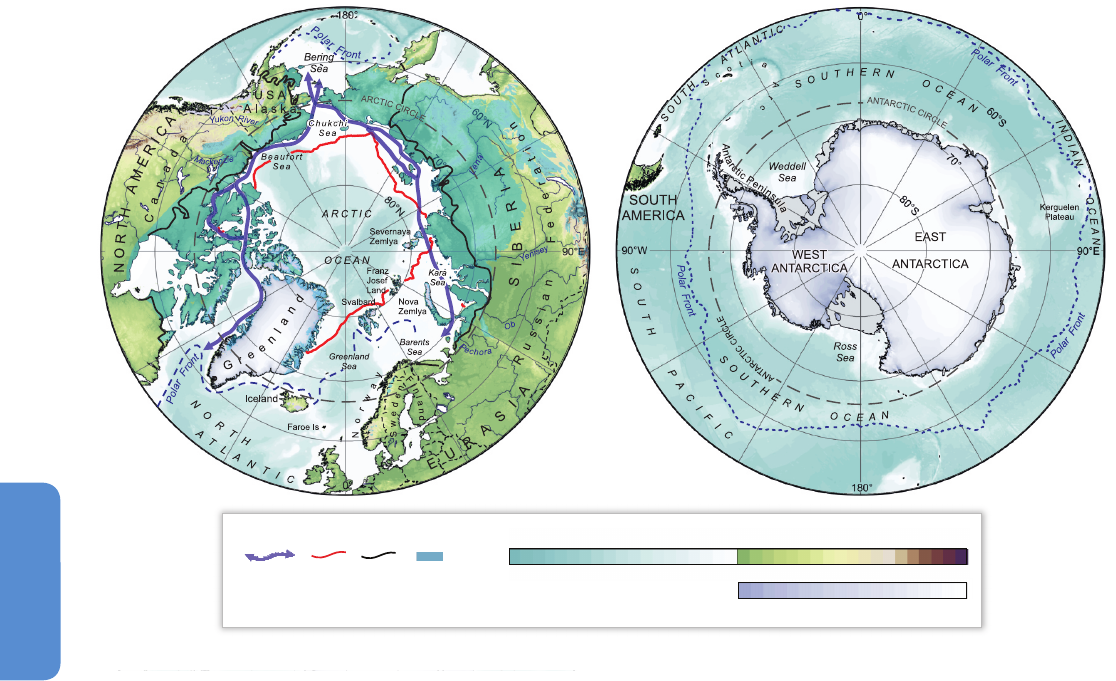
Sea-bed depths
Shipping
route
September
sea ice
Tree
line
Continuous
permafrost
Glaciated land
Non-glaciated land
3000+
1000
500
–7000
–1000
–3000–5000
Depth (m)
Height (m)
–200
200
2000
Figure 28-1| Location maps of the north and south polar regions (courtesy of P. Fretwell, British Antarctic Survey).

1573
Polar Regions Chapter 28
28
r
iver flow (1936–1999; Peterson et al., 2002) could not, for a similar
period (1951–2000), be attributed with certainty to precipitation
changes (Milliman et al., 2008) but has been, including more recent
extreme increases (2007), attributed to enhanced poleward atmospheric
moisture transport (Zhang et al., 2013). By contrast, decreased flow in
high-latitude Canadian rivers (1964–2000; average –10%) does match
that for precipitation (Déry and Wood, 2005). Recent data (1977–2007)
for 19 circumpolar rivers also indicate an area-weighted average increase
of +9.8% (–17.1 to 47.0%; Overeem and Syvitski, 2010) accompanied
by shifts in flow timing, with May snowmelt increasing (avg. 66%) but
flow in the subsequent month of peak discharge decreasing (~7%).
Across the Russian Arctic, dates of spring maximum discharge have also
started to occur earlier, particularly in the most recent (1960–2001)
period analyzed (average –5 days; range for four regions +0.2 to –7.1
days), but no consistent trend exists for magnitude (average –1%; range
+21 to –24%; Shiklomanov et al., 2007). Earlier timing was most pro-
nounced in eastern, colder continental climates, where increases in air
temperature have been identified as the dominant control (Tan et al.,
2011).
Increases have also occurred in winter low flows for many Eurasian and
North American rivers (primarily in the late 20th century; Smith et al.,
2007; Walvoord and Striegl, 2007; St. Jacques and Sauchyn, 2009; Ye et
al., 2009), the key exceptions being decreases in eastern North America
and unchanged flow in small basins of eastern Eurasia (Rennermalm
et al., 2010). Most such studies suggest permafrost thaw (WGI AR5
Chapter 4) has increased winter flow, whereas others suggest increases
in net winter precipitation minus evapotranspiration (Rawlins et al.,
2009a,b; Landerer et al., 2010). Insufficient precipitation stations preclude
deciphering the relative importance of these factors (WGI AR5 Section
2.5.1).
The surface-water temperatures of large water bodies has warmed
(1985–2009; Schneider and Hook, 2010), particularly for mid- and high
latitudes of the Northern Hemisphere, with spatial patterns generally
matching those for air temperature. Where water bodies warmed more
rapidly than air temperature, decreasing ice cover was suggested as
enhancing radiative warming. Paleolimnological evidence indicates that
the highest primary productivity was associated with warm, ice-free
summer conditions and the lowest with periods of perennial ice (Melles
et al., 2007). Increasing water temperatures affect planktonic and benthic
biomass and lead to changes in species composition (Christoffersen et
al., 2008; Heino et al., 2009, Jansson et al., 2010). Reduced ice cover
with higher air temperatures and evaporation are responsible for the
late-20th to early-21st century desiccation of some Arctic ponds (Smol
and Douglas, 2007).
Changes have occurred in the size and number of permafrost lakes over
the last half-century (Hinkel et al., 2007; Marsh et al., 2009), but their
patterns and rates of change are not consistent because of differing
thawing states, variations in warming, and effects of human activities
(Hinket et al., 2007; Prowse and Brown, 2010a). Thawing permafrost
affects the biogeochemistry of water entering lakes and rivers (Frey and
McClelland, 2009; Kokelj et al., 2009) and their ecological structure and
function (Lantz and Kokelj, 2008; Thompson et al., 2008; Mesquita et
al., 2010), such as enhancing eutrophication by a shift from pelagic to
benthic-dominated production (Thompson et al., 2012).
T
he aquatic ecosystem health and biodiversity of northern deltas is
dependent on combined changes in the elevation of spring river ice-
jam floods and sea level (Lesack and Marsh, 2007, 2010). Diminishing
ice shelves (last half-century) have also caused a decline in the number
of freshwater epishelf lakes that develop behind them (Veillette et al.,
2008; Vincent et al., 2009). Although such biophysical dependencies
have been established, temporal trends in such river-delta and epishelf
lake impacts and their linkages to changing climate remain to be
quantified precisely.
An interplay of freshwater-marine conditions also affects the timing,
growth, run size, and distribution of several Arctic freshwater and
anadromous fish. Key examples include the timing of marine exit of Yukon
River Chinook salmon (Oncorhynchus tshawytscha; 1961–2009) varied
with air and sea temperatures and sea ice cover (Mundy and Evenson,
2011); the growth of young-of-year Arctic cisco (Coregonus autumnalis;
1978–2004) varied in response to lagged sea ice concentration and
Mackenzie River discharge, also indicating that decreased sea ice
concentration and increased river discharge enhanced marine primary
production, leading to more favorable foraging conditions (von Biela et
al., 2011); and factors that influence the water level and freshening of
rivers, as well as the strength, duration, and directions of prevailing
coastal winds, affect survival of anadromous fishes during coastal
migration and their subsequent run size (Fechhelm et al., 2007).
28.2.1.2. Antarctic
Biota of Antarctic freshwater systems (lakes, ponds, short streams,
and seasonally wetted areas) are dominated by benthic microbial
communities of cyanobacteria and green algae in a simple food web.
Mosses occur in some continental lakes with higher plants absent.
Planktonic ecosystems are typically depauperate and include small algae,
bacteria, and colorless flagellates, with few metazoans and no fish
(Quesada and Velázquez, 2012). Recent compilations of single-year data
sets have reinforced previous conclusions on the changing freshwater
habitats in Antarctica (Verleyen et al., 2012). In regions where the climate
has warmed, the physical impacts on aquatic ecosystems include loss of
ice and perennial snow cover, increasing periods of seasonal open water,
increased water column temperatures, and changes in water column
stratification. In some areas, a negative water balance has occurred as
a result of increased temperature and changes in wind strength driving
enhanced evaporation and sublimation and leading to increased salinity
in lakes in recent decades (Hodgson et al., 2006a). In other areas,
especially glacial forelands, increased temperatures have led to greater
volumes of seasonal meltwater in streams and lakes together with
increased nutrient fluxes (high confidence). In both cases, the balance
between precipitation and evaporation can have detectable effects on
lake ecosystems (medium confidence) through changes in water body
volume and lake chemistry (Lyons et al., 2006; Quesada et al., 2006).
Non-dilute lakes with a low lake depth to surface area ratio are most
susceptible to interannual and inter-decadal variability in the water
balance, as measured by changes in specific conductance (high confidence;
Verleyen et al., 2012). Warming in the northwestern Antarctic Peninsula
region has resulted in permafrost degradation in the last approximately
50 years, impacting surface geomorphology and hydrology (Bockheim
et al., 2013) with the potential to increase soil biomass.

1574
Chapter 28 Polar Regions
28
28.2.2. Oceanography and Marine Ecosystems
28.2.2.1. Arctic
2
8.2.2.1.1. Marine plankton, fish, and other invertebrates
WGI documented the expected physical and chemical changes that will
occur in Arctic marine ecosystems (WGI AR5 Chapters 4, 6, 11). Naturally
occurring interannual, decadal, and multi-decadal variations in climate
will continue to influence the Arctic Ocean and its neighboring high-
latitude seas (Chapter 5). In recent years (2007–2012), ocean conditions
in the Bering Sea have been cold (Stabeno et al., 2012a), while the
Barents Sea has been warm (Lind and Ingvaldsen, 2012).
In this section, we build on previous reviews of observed species responses
to climate (Wassman et al., 2011) to summarize the current evidence
of the impact of physical and chemical changes in marine systems on
the phenology, spatial distribution, and production of Arctic marine
species. For each type of response, the implications for phytoplankton,
zooplankton, fish, and shellfish are discussed. The implications of these
changes on marine ecosystem structure and function will be the result
of the synergistic effects of all three types of biological responses.
Phenological response
The timing of spring phytoplankton blooms is a function of seasonal light,
hydrographic conditions, and the timing of sea ice breakup (Wassman,
2011). In addition to the open water phytoplankton bloom, potentially
large ice algal blooms can form under the sea ice (Arrigo, 2012). During
the period 1997–2009, a trend toward earlier phytoplankton blooms
was detected in approximately 11% of the area of the Arctic Ocean
(Kahru et al., 2011). This advanced timing of annual phytoplankton
blooms coincided with decreased sea ice concentration in early summer.
Brown and Arrigo (2013) studied the timing and intensity of spring
blooms in the Bering Sea from 1997 to 2010 and found that in northern
regions sea ice consistently retreated in late spring and was associated
with ice-edge blooms, whereas in the southern regions the timing of
sea ice retreat varied, with ice-edge blooms associated with late ice
retreat, and open water blooms associated with early ice retreat. Given
the short time series and limited studies, there is medium confidence
that climate variability and change has altered the timing and the
duration of phytoplankton production.
The life cycles of calanoid copepods in the Arctic Ocean and Barents Sea
are timed to utilize ice algal and phytoplankton blooms (Falk-Petersen
et al., 2009; Søreide et al., 2010; Darnis et al., 2012). Based on a
synthesis of existing data, Hunt, Jr. et al. (2011) hypothesized that, in
the southeastern Bering Sea, ocean conditions and the timing of sea
ice retreat influences the species composition of dominant zooplankton,
with lipid-rich copepods being more abundant in cold years.
There is ample evidence that the timing of spawning and hatching of
some fish and shellfish is aligned to match larval emergence with
seasonal increases in prey availability (Gjosaeter et al., 2009; Vikebø et
al., 2010; Bouchard and Fortier, 2011; Drinkwater et al., 2011). These
regional phenological adjustments to local conditions occurred over
m
any generations (Ormseth and Norcross, 2009; Geffen et al., 2011;
Kristiansen et al., 2011). There is medium to high confidence that climate-
induced disruptions in this synchrony can result in increased larval or
juvenile mortality or changes in the condition factor of fish and shellfish
species in the Arctic marine ecosystems.
O
bserved spatial shifts
Spatial heterogeneity in primary production has been observed (Lee et
al., 2010; Grebmeier, 2012). Simulation modeling studies show that
spatial differences in the abundance of four species of copepod can be
explained by regional differences in the duration of the growing season
and temperature (Ji et al., 2012). Retrospective studies based on surveys
from 1952 to 2005 in the Barents Sea revealed that changes in the
species composition, abundance, and distribution of euphausiids were
related to climate-related changes in oceanographic conditions (Zhukova
et al., 2009).
Retrospective analysis of observed shifts in the spatial distribution of
fish and shellfish species along latitudinal and depth gradients showed
observed spatial shifts were consistent with expected responses of
species to climate change (Simpson et al., 2011; Poloczanska et al.,
2013; see also Box CC-MB). Retrospective studies from the Bering Sea,
Barents Sea, and the northeast Atlantic Ocean and Icelandic waters
showed that fish shift their spatial distribution in response to climate
variability (i.e., interannual, decadal, or multi-decadal changes in ocean
temperature; Mueter and Litzow, 2008; Sundby and Nakken, 2008;
Hátún et al., 2009; Valdimarsson et al., 2012; Kotwicki and Lauth, 2013).
There are limits to the movement potential of some species. Vulnerability
assessments indicate that the movement of some sub-Arctic fish and
shellfish species into the Arctic Ocean may be impeded by the presence
of water temperatures on the shelves that fall below their thermal
tolerances (Hollowed et al., 2013; Hunt, Jr. et al., 2013). Coupled
biophysical models have reproduced the observed spatial dynamics of
some the species in the Bering and Barents Seas, and are being used to
explain the role of climate variability and change on the distribution
and abundance of some species (Huse and Ellingsen, 2008; Parada et
al., 2010). In summary, there is medium to high confidence based on
observations and modeling that some fish and shellfish have shifted their
distribution in response to climate impacts on the spatial distribution
and volume of suitable habitat.
Observed variations in production
Seasonal patterns in light, sea ice cover, freshwater input, stratification,
and nutrient exchange act in concert to produce temporal cycles of ice
algal and phytoplankton production in Arctic marine ecosystems
(Perrette et al., 2011; Wassmann, 2011; Tremblay et al., 2012). Satellite
observations and model estimates for the period 1988–2007 showed
that phytoplankton productivity increased in the Arctic Ocean in response
to a downward trend in the extent of summer sea ice (Zhang et al.,
2010). Satellite data provided evidence of a 20% increase in annual net
primary production in the Arctic Ocean between 1998 and 2009 in
response to extended ice-free periods (Arrigo and van Dijken, 2011).
Regional trends in primary production will differ in response to the

1575
Polar Regions Chapter 28
28
a
mount of open water area in summer (Arrigo and van Dijken, 2011).
Other studies showed gross primary production increased with increasing
air temperature in the Arctic Basin and Eurasian shelves (Slagstad et
al., 2011). A recent 5-year study (2004–2008) in the Canada Basin
showed that smaller phytoplankton densities were higher than larger
phytoplankton densities in years when sea surface temperatures (SSTs)
were warmer, the water column was more stratified, and nutrients were
more depleted during the Arctic summer (Li et al., 2009; Morán et al.,
2010). Additional observations will help to resolve observed differences
between in situ and satellite-derived estimates of primary production
(Matrai et al., 2013). In conclusion, based on recent observations and
modeling, there is medium to high confidence that primary production
has increased in the Arctic Ocean in response to changes in climate
and its impact on the duration and areal extent of ice-free periods in
summer.
Regional differences in zooplankton production have been observed.
During a period of ocean warming (1984–2010), Dalpadado et al. (2012)
observed an increase in the biomass of lipid-rich euphausiids in the
Barents Sea and relatively stable levels of biomass and production of
Calanus finmarchicus. In the Bering Sea, observations over the most
recent decade in the southeast Bering Sea showed C. marshallae were
more abundant in cold than in warm years (Coyle et al., 2011).
There is strong evidence that climate variability impacts the year-class
strength of Arctic marine fish and shellfish through its influence on
predation risk; the quality, quantity, and availability of prey; and
reproductive success (Mueter et al., 2007; Bakun 2010; Drinkwater et
al., 2010). Regional differences in the species responses to climate
change will be a function of the exposure of the species to changing
environmental conditions, the sensitivity of the species to these changes
(Beaugrand and Kirby, 2010), and the abilities of species to adapt to
changing conditions (Pörtner and Peck, 2010; Donelson et al., 2011).
There is high confidence that shifts in ocean conditions have impacted
the abundance of fish and shellfish in Arctic regions. Observed trends in
the abundance of commercial fish and shellfish may also be influenced
by historical patterns of exploitation (Vert-pre et al., 2013).
28.2.2.1.2. Marine mammals, polar bears, and seabirds
Studies on responses of Arctic and subarctic marine mammals to climate
change are limited and vary according to insight into their habitat
requirements and trophic relationships (Laidre et al., 2008). Many Arctic
and sub-Arctic marine mammals are highly specialized, have long life
spans, and are poorly adapted to rapid environmental change (Moore
and Huntington, 2008), and changes may be delayed until significant
sea ice loss has occurred (Freitas et al., 2008; Laidre et al., 2008).
Climate change effects on Arctic and sub-Arctic marine mammal
species will vary by life history, distribution, and habitat specificity (high
confidence). Climate change will improve conditions for a few species,
have minor negative effects for others, and some will suffer major
negative effects (Laidre et al., 2008; Ragen et al., 2008). Climate change
resilience will vary and some ice-obligate species should survive in
regions with sufficient ice and some may adapt to ice-free conditions
(Moore and Huntington, 2008). Less ice-dependent species may be more
a
daptable but an increase in seasonally migrant species could increase
competition (Moore and Huntington, 2008).
Climate change vulnerability was associated with feeding specialization,
ice dependence, and ice reliance for prey access and predator avoidance
(Laidre et al., 2008). There is medium agreement on which species’ life
histories are most vulnerable. Hooded seals (Cystophora cristata) and
narwhal (Monodon monoceros) were identified as most at risk and
ringed seals (Pusa hispida) and bearded seals (Erignathus barbatus) as
least sensitive (Laidre et al., 2008). Kovacs et al. (2010) shared concern
for hooded seals and narwhal but had concerns for ringed seals and
bearded seals. Narwhal may have limited ability to respond to habitat
alteration (Williams et al., 2011). Species that spend only part of the
year in the Arctic (e.g., gray whale (Eschrichtius robustus), killer whale
(Orcinus orca)) may benefit from reduced ice (Laidre et al., 2008; Moore,
2008; Higdon and Ferguson, 2009; Matthews et al., 2011; Ferguson et
al., 2012). Killer whale expansion into the Arctic could cause a trophic
cascade (Higdon and Ferguson, 2009), although there is limited evidence
at this time.
There is limited evidence although medium agreement that generalists
and pelagic feeding species may benefit from increased marine productivity
from reduced ice while benthic feeding species near continental shelf
habitats may do poorly (Bluhm and Gradinger, 2008). There is limited
evidence but high agreement that dietary or habitat specialists will do
poorly with reduced ice. Reduction of summer/autumn ice was the primary
extrinsic factor affecting Pacific walrus (Odobenus rosmarus), with
predictions of distribution changes, reduced calf recruitment, and longer
term predictions of high extinction probability (Cooper et al., 2006;
MacCracken, 2012). Summer ice retreat may make migration to such
habitats energetically unprofitable for ringed seals (Freitas et al., 2008).
Ice loss threatens Baltic ringed seals (Kovacs and Lydersen, 2008). In
Hudson Bay, earlier spring break-up and changes in snow cover over
lairs have reduced ringed seal recruitment (Ferguson et al., 2005).
Changes in snowfall over the 21st century were projected to reduce
ringed seal habitat for lairs by 70% (Hezel et al., 2012). Similarly, harp
seal (Pagophilus groenlandicus) breeding habitat was affected by
changing ice conditions that could reduce pup survival (Bajzak et al.,
2011). Although there is limited evidence, there are concerns that climate
change may cause indirect effects on Arctic marine mammals’ health
(e.g., pathogen transmission, food web changes, toxic chemical exposure,
shipping, and development; Burek et al., 2008).
Empirical studies provide direct insight into the mechanisms of climate
change impact on polar bears (Ursus maritimus) but modeling allows
predictive capacity (Amstrup et al., 2010; Hunter et al., 2010; Durner et
al., 2011; Castro de la Guardia et al., 2013).
Polar bears are highly specialized and use annual ice over the continental
shelves as their preferred habitat (Durner et al., 2009; Miller et al., 2012).
The recent and projected loss of annual ice over continental shelves,
decreased ice duration, decreased ice thickness, and habitat fragmentation
are causing reduced food intake, increased energy expenditure, and
increased fasting in polar bears (high confidence; Stirling and Parkinson,
2006; Regehr et al., 2007; Durner et al., 2009; Amstrup et al., 2010;
Hunter et al., 2010; Derocher et al., 2011; Rode et al., 2012; Sahanatien
and Derocher, 2012; Castro de la Guardia et al., 2013).

1576
Chapter 28 Polar Regions
28
S
ubpopulation response varies geographically. Only 2 of the 19
subpopulations—Western Hudson Bay (Regehr et al., 2007) and the
southern Beaufort Sea (Regehr et al., 2010; Rode et al., 2010a)—have
data series adequate for clear identification of abundance effects related
to climate change. Many other subpopulations show characteristics
associated with decline but some remain stable. Declining ice is causing
lower body condition, reduced individual growth rates, lower fasting
endurance, lower reproductive rates, and lower survival (high confidence;
Regehr et al., 2007, 2010; Rode et al., 2010a, 2012; Molnar et al., 2011).
Condition is a precursor to demographic change (very high confidence;
Hunter et al., 2010; Regehr et al., 2010; Rode et al., 2010a; Robinson et
al., 2011). The decline in the subpopulation in Western Hudson Bay by
21% between 1987 and 2004 was related to climate change (medium
confidence; Regehr et al., 2007). Replacement of multi-year ice by
annual ice could increase polar bear habitat (low confidence; Derocher
et al., 2004). Increasing the distance to multi-year ice and terrestrial
refugia at maximal melt may result in drowning, cub mortality, and
increased energetic costs (Monnett and Gleason, 2006; Durner et al.,
2011; Pagano et al., 2012). There is robust evidence of changes in sea
ice conditions changing polar bear distribution including den areas (high
confidence; Fischbach et al., 2007; Schliebe et al., 2008; Gleason and
Rode, 2009; Towns et al., 2010; Derocher et al., 2011). The number of
human-bear interactions is projected to increase with warming (high
confidence; Stirling and Parkinson, 2006; Towns et al., 2009).
Use of terrestrial resources by polar bears was suggested as adaptive
(Dyck et al., 2007, 2008; Dyck and Romberg, 2007; Armstrong et al.,
2008; Dyck and Kebreab, 2009; Rockwell and Gormezano, 2009; Smith
et al., 2010). Polar bears cannot adapt to terrestrial foods (Stirling et
al., 2008b; Amstrup et al., 2009; Rode et al., 2010b; Slater et al., 2010),
and will most likely not be able to adapt to climate change and reduced
sea ice extent (very high confidence). Changing ice conditions are linked
to cannibalism (Amstrup et al., 2006), altered feeding (Cherry et al.,
2009), unusual hunting behavior (Stirling et al., 2008a), and diet change
(Iverson et al., 2006; Thiemann et al., 2008) (medium confidence).
Upwelling or subsurface convergence areas found in frontal zones and
eddies, and the marginal ice zone, are associated with high marine
productivity important to Arctic seabirds (e.g., Irons et al., 2008). Long-
term or permanent shifts in convergence areas and the marginal ice-
edge zone induced by climate change may cause mismatch between
the timing of breeding and the peak in food availability, and thus
potentially have strong negative impacts on seabird populations (medium
confidence; Gaston et al., 2005, 2009; Moline et al., 2008; Grémillet and
Boulinier, 2009).
The contrasting results from the relatively few studies of impacts of
climate change on Arctic seabirds demonstrate that future impacts will
be highly variable between species and between populations of the
same species (medium confidence). Retreating sea ice and increasing
SSTs have favored some species and disadvantaged others (Gaston et
al., 2005; Byrd et al., 2008; Irons et al., 2008; Karnovsky et al., 2010;
Fredriksen et al., 2013). Some species of seabirds respond to a wide
range of sea surface temperatures via plasticity of their foraging
behavior, allowing them to maintain their fitness levels (Grémillet et al.,
2012). Phenological changes and changes in productivity of some
breeding colonies have been observed (Byrd et al., 2008; Gaston and
W
oo, 2008; Moe et al., 2009). Negative trends in population size,
observed over the last few decades for several species of widespread
Arctic seabirds, may be related to over-harvesting and pollution as well
as climate change effects (Gaston, 2011). For those species whose
distribution is limited by sea ice and cold water, polar warming could
be beneficial (Mehlum, 2012).
A major ecosystem shift in the northern Bering Sea starting in the mid-
1990s caused by increased temperatures and reduced sea ice cover had
a negative impact on benthic prey for diving birds, and these populations
have declined in the area (Grebmeier et al., 2006). More recently, the
Bering Sea has turned colder again.
28.2.2.2. Antarctica
Productivity and food web dynamics in the Southern Ocean are dominated
by the extreme seasonal fluctuations of irradiance and the dynamics of
sea ice, along with temperature, carbonate chemistry, and vertical
mixing (Massom and Stammerjohn, 2010; Boyd et al., 2012; Murphy et
al., 2012a). Moreover, there is large-scale regional variability in habitats
(Grant et al., 2006) and their responses to climate change. Antarctic
krill, Euphausia superba (hereafter, krill), is the dominant consumer,
eating diatoms, and, in turn, is the main prey of fish, squid, marine
mammals, and seabirds. Krill is dominant from the Bellingshausen Sea
east through to the Weddell Sea and the Atlantic sector of the Southern
Ocean (Rogers et al., 2012). In the East Indian and southwest Pacific
sectors of the Southern Ocean, the krill-dominated system lies to the
south of the Southern Boundary of the Antarctic Circumpolar Current
(Nicol et al., 2000a,b) while to the north copepods and myctophid fish
are most important (Rogers et al., 2012). Further west, where the
Weddell Sea exerts an influence, krill are found as far north as the Sub-
Antarctic Circumpolar Current Front (Jarvis et al., 2010). Where sea ice
dominates for most of the year, ice-obligate species (e.g., Euphausia
crystallorophias and Peluragramma antarcticum) are most important
(Smith et al., 2007).
Few studies were available in AR4 to document and validate the
changes in these systems resulting from climate change. Those studies
reported increasing abundance of benthic sponges and their predators,
declining populations of krill, Adélie and emperor penguins, and Weddell
seals, and a possible increase in salps, noting some regional differences
in these trends. The importance of climate processes in generating these
changes could not be distinguished from the indirect consequences of
the recovery of whale and seal populations from past over-exploitation
(Trathan and Reid, 2009; Murphy et al., 2012a,b).
28.2.2.2.1. Marine plankton, krill, fish, and other invertebrates
Distributions of phytoplankton and zooplankton have moved south with
the frontal systems (Hinz et al., 2012; Mackey et al., 2012), including
range expansion into the Southern Ocean from the north by the
coccolithophorid Emiliania huxleyi (Cubillos et al., 2007) and the red-
tide dinoflagellate Noctiluca scintillans (McLeod et al., 2012) (medium
confidence). There is insufficient evidence to determine whether other
range shifts are occurring.

1577
Polar Regions Chapter 28
28
C
ollapsing ice shelves are altering the dynamics of benthic assemblages
by exposing areas previously covered by ice shelves, allowing increased
primary production and establishment of new assemblages (e.g., collapse
of the Larson A/B ice shelves) (medium confidence; Peck et al., 2009;
Gutt et al., 2011). More icebergs are grounding, causing changes in
local oceanography and declining productivity that consequently affects
productivity of benthic assemblages (low confidence; Thrush and
Cummings, 2011). Iceberg scour on shallow banks is also increasing,
disrupting resident benthic assemblages (medium confidence; Barnes
and Souster, 2011; Gutt et al., 2011).
Primary production is changing regionally in response to changes in sea
ice, glacial melt, and oceanographic features (medium confidence;
Arrigo et al., 2008; Boyd et al., 2012). Off the west Antarctic Peninsula,
phytoplankton stocks and productivity have decreased north of 63°S,
but increased south of 63°S (high confidence; Montes-Hugo et al., 2009;
Chapter 6). This study (based on time series of satellite-derived and
measured chlorophyll concentrations) also indicated a change from
diatom-dominated assemblages to ones dominated by smaller
phytoplankton (Montes-Hugo et al., 2009). The reduced productivity in
the north may be tempered by increased inputs of iron through changes
to ocean processes in the region (low confidence; Dinniman et al., 2012).
Since the 1980s, Antarctic krill densities have declined in the Scotia Sea
(Atkinson et al., 2004), in parallel with regional declines in the extent
and duration of winter sea ice (Flores et al., 2012). Uncertainty remains
over changes in the krill population because this decline was observed
using net samples and is not reflected in acoustic abundance time series
(Nicol and Brierley, 2010); the observed changes in krill density may
have been partly a result of changes in distribution (Murphy et al.,
2007). Nevertheless, given its dependence on sea ice (Nicol et al., 2008),
the krill population may already have changed and will be subject to
further alterations (high confidence).
The response of krill populations is probably a complex response to
multiple stressors. Decreases in recruitment of post-larval krill across
the Scotia Sea have been linked to declines in sea ice extent in the
Antarctic Peninsula region (medium confidence; Wiedenmann et al., 2009)
but these declines may have been offset by increased growth arising
from increased water temperature in that area (Wiedenmann et al.,
2008). However, near South Georgia krill productivity may have declined
as a result of the increased metabolic costs of increasing temperatures
(low confidence; Hill et al., 2013). The combined effects of changing sea
ice, temperature, and food have not been investigated.
28.2.2.2.2. Marine mammals and seabirds
In general, many Southern Ocean seals and seabirds exhibit strong
relationships to a variety of climate indices, and many of these relationships
are negative to warmer conditions (low confidence; Trathan et al., 2007;
Barbraud et al., 2012; Forcada et al., 2012). Regional variations in climate
change impacts on habitats and food will result in a mix of direct and
indirect effects on these species. For example, Adélie penguin colonies
are declining in recent decades throughout the Antarctic Peninsula while
the reduction in chinstrap penguins is more regional (Lynch et al., 2012)
and related to reductions in krill availability (Lima and Estay, 2013). In
c
ontrast, gentoo penguins are increasing in that region and expanding
south (high confidence; Lynch et al., 2012). This may be explained by the
reduced sea ice habitats and krill availability in the north, resulting in a
southward shift of krill predators, particularly those dependent on sea
ice (Forcada et al., 2012) and the replacement of these predators in the
north by species that do not depend on sea ice, such as gentoo penguins
and elephant seals (low confidence; Costa et al., 2010; Trivelpiece et al.,
2011; Ducklow et al., 2012; Murphy et al., 2013). A contrasting situation
is in the Ross Sea, where Adélie penguin populations have increased
(Smith, Jr. et al., 2012). The mechanisms driving these changes are
currently under review and may be more than simply sea ice (Lynch et
al., 2012; Melbourne-Thomas et al., 2013). For example, too much or
too little sea ice may have negative effects on the demography of Adélie
and emperor penguins (see Barbraud et al., 2012, for review). Also,
increased snow precipitation that accumulates in breeding colonies can
decrease survival of chicks of Adélie penguins when accompanied by
reduced food supply (Chapman et al., 2011).
Changes elsewhere are less well known. Some emperor penguin
colonies have decreased in recent decades (low confidence; Barbraud
et al., 2008; Jenouvrier et al., 2009), and one breeding site has been
recorded as having been vacated (Trathan et al., 2011). However, there
is insufficient evidence to make a global assessment of their current
trend. In the sub-Antarctic of the Indian sector, reductions in seal and
seabird populations may indicate a region-wide shift to a system with
lower productivity (low confidence; Weimerskirch et al., 2003; Jenouvrier
et al., 2005a,b) but commercial fishing activities may also play a role.
Where frontal systems are shifting south, productive foraging areas also
move to higher latitudes. In the Indian sector, this is thought to be
causing declines in king penguin colonies on sub-Antarctic islands (low
confidence; Péron et al., 2010), while the shift in wind patterns may be
causing changes to the demography of albatross (low confidence;
Weimerskirch et al., 2012).
As identified in the WGII AR4, some species’ populations may suffer as a
result of fisheries while others are recovering from past over-exploitation,
either of which may confound interpretation of the response of these
species and their food webs to climate change. The recovery of Antarctic
fur seals on some sub-Antarctic islands has been well documented, and
their populations may now be competing with krill-eating macaroni
penguins (Trathan et al., 2012). More recently, there has been confirmation
that populations of some Antarctic whales are recovering, such as
humpbacks (Nicol et al., 2008; Zerbini et al., 2010), suggesting that food
is currently not limiting. In contrast, a number of albatross and petrel
populations are declining as a result of incidental mortality in longline
fisheries in southern and temperate waters where these birds forage
(Croxall et al., 2012).
28.2.3. Terrestrial Ecosystems
28.2.3.1. Arctic
Arctic terrestrial ecosystems have undergone dramatic changes
throughout the late Pleistocene and Holocene (last 130,000 years),
mainly driven by natural climate change. Significant altitudinal and
1578
Chapter 28 Polar Regions
28
l
atitudinal advances and retreats in tree line have been common, animal
species have gone extinct, and animal populations have fluctuated
significantly throughout this period (e.g., Lorenzen et al., 2011; Salonen
et al., 2011; Mamet and Kershaw, 2012).
28.2.3.1.1. Phenology
Phenological responses attributable to warming are apparent in most
Arctic terrestrial ecosystems (medium confidence). They vary from earlier
onset and later end of season in western Arctic Russia (Zeng et al., 2013),
to little overall trend in plant phenology in the Swedish sub-Arctic
(Callaghan et al., 2010), to dramatic earlier onset of phenophases in
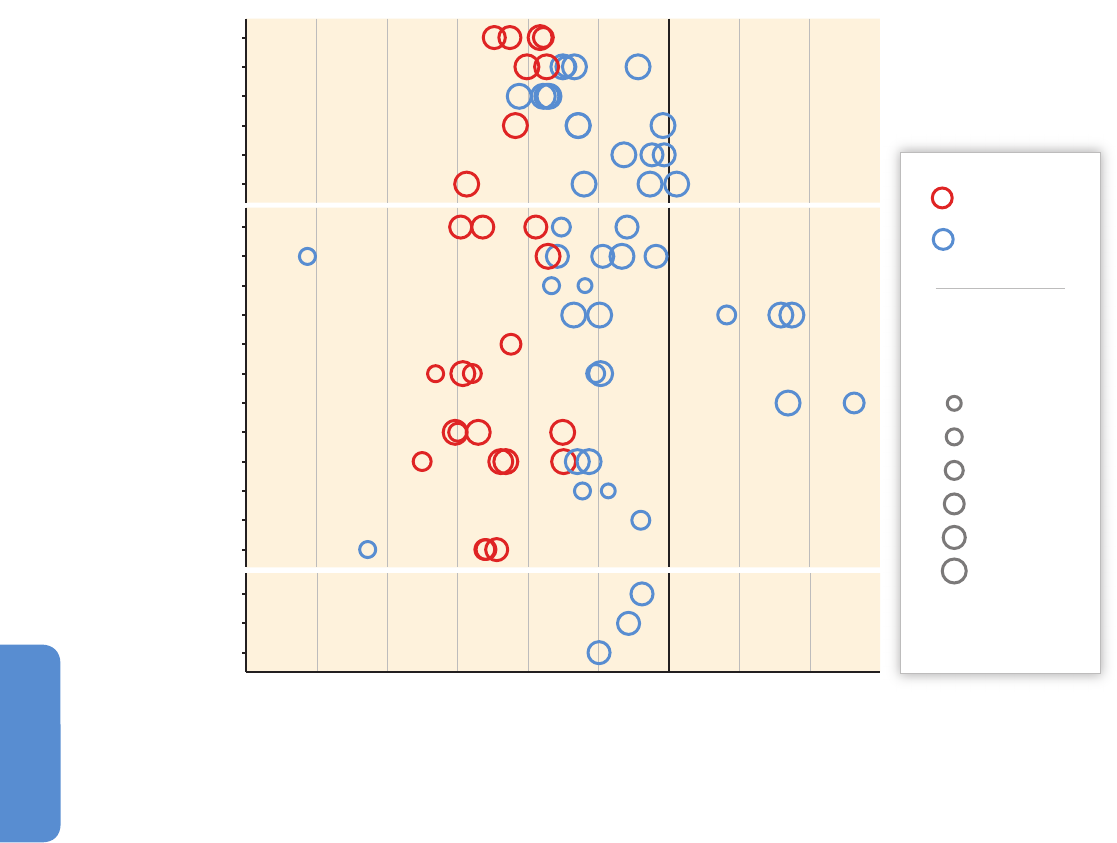
Greenland (Høye et al., 2007; Post et al., 2009a; Callaghan et al., 2011a;
see Figure 28-2).
28.2.3.1.2. Vegetation
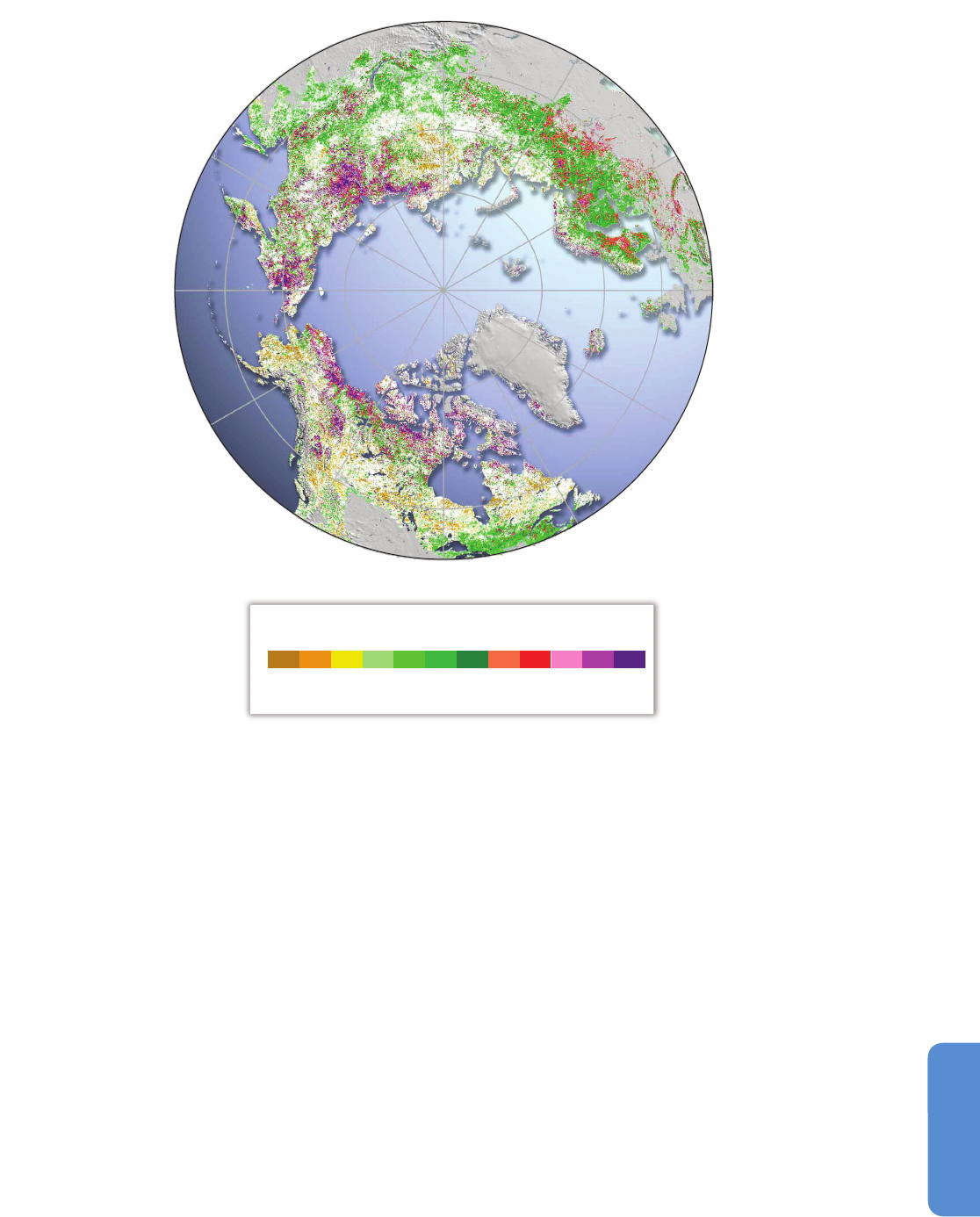
The latest assessment of changes in Normalized Difference Vegetation
Index (NDVI), a proxy for plant productivity, from satellite observations
between 1982 and 2012 shows that about a third of the Pan-Arctic has
s
ubstantially greened, less than 4% browned, and more than 57% did
not change significantly (Xu et al., 2013; Figure 28-3). The greatest
increases reported in recent years were in the North American high Arctic,
along the Beaufort Sea and the east European Arctic (Zhang et al., 2008;
Pouliot et al., 2009; Bhatt et al., 2010; Forbes et al., 2010; Walker et al.,
2011; Epstein et al., 2012; Macias-Fauria et al., 2012; Xu et al., 2013).
The positive trends in NDVI are associated with increases in the summer
warmth index (sum of the monthly mean temperatures above freezing
expressed as degrees Celsius per month) that have increased on average
by 5°C per month for the Arctic as a whole (Xu et al., 2013). However,
the even greater 10°C to 12°C per month increase for the land adjacent
to the Chukchi and Bering Seas (Figure 28-3) was associated with
decreases in NDVI. On the Yamal Peninsula in Russia the pattern of NDVI
is partly due to surface disturbance, such as landslide activity (Walker
et al., 2009). Small rodent cycles reduce NDVI in sub-Arctic Sweden, by
decreasing biomass and changing plant species composition (Olofsson
et al., 2012). The changing NDVI signal should therefore generally be
interpreted with care.
In common with tree line trees and herbs, theabundance and biomass
of deciduous shrubs and graminoids (grasses and grass-like plants) have
–60 –50 –40 –30 –20 –10
0
10 20 30
Cassiope tetragona
Papaver radicatum
Salix arctica
Saxifraga oppositifolia
Silene acaulis
Acari*
5 years
Statistically significant
Statistically insignificant
6 years
7 years
8 years
9 years
10 years
Chironomidae
Coc coidea
Collembola*
Culicidae
Ic hneumonidae
Linyphiidae*
Lycosidae
Muscidae
Nymphalidae
Phoridae
Sciaridae
Dunlin
Sanderling
Ruddy turnstone
Dryas sp.
Plants
Arthropods
Birds
Mean phenological change (days per decade)
Number of years of data available
for the calculation of each
temporal trend
* = likely biased
Figure 28-2 | Temporal change in onset of flowering (plants), median date of emergence (arthropods), and clutch initiation dates (birds) estimated from weekly sampling in
permanents plots (plants and arthropods) and near-daily surveys through the breeding period in a 19 km
2
census area (birds) during 1996–2005 in high-Arctic Greenland. Trends
based on 5 to 10 years of observations are red circles when statistically significant and otherwise blue. Trends in arthropod taxa marked by asterisks (*) are likely to be biased
(Høye et al., 2007).
1579
Polar Regions Chapter 28
28
increased substantially in certain parts of the Arctic tundra in recent
years, but remained stable or decreased in others (very high confidence).
Attribution for the increases and decreases in deciduous shrubs and
graminoids is heterogeneous, with drivers varying among different
regions (very likely), including Arctic warming, differences in herbivory,
industrial development, legacies from past land use, and changes in
moisture (Post and Pedersen, 2008; Forbes et al., 2009, 2010; Kitti et
al., 2009; Olofsson et al., 2009; Callaghan et al., 2011b, 2013; Kumpula
et al., 2011, 2012; Myers-Smith et al., 2011;Elmendorf et al., 2012b;
Gamon et al., 2013).
Shrubs have generally expanded their ranges and/or growth over the
last 20 years (Danby and Hik, 2007; Hudson and Henry, 2009; Forbes et
al., 2010; Hallinger et al., 2010; Callaghan et al., 2011b; Hedenås et al.,
2011; Hill and Henry, 2011; Myers-Smith et al., 2011a,b; Rundqvist et
al., 2011; Elmendorf et al., 2012a,b; Macias-Fauria et al., 2012), and
have varied from dramatic, that is, 200% area increase in study plots
(Rundqvist et al., 2011) in sub-Arctic Sweden, to early invasion of a fell
field community on west Greenland by low shrubs (Callaghan et al.,
2011a).
A synthesis (61 sites; Elmendorf et al., 2012a) of experimental warming
studies of up to 20 years duration in tundra sites worldwide showed,
overall, increased growth of deciduous shrubs and graminoids, decreased
cover of mosses and lichens, and decreased species diversity and
evenness. Elmendorf et al. (2012a) point out that the groups that
increased most in abundance under simulated warming were graminoids
in cold regions and primarily shrubs in warm regions of the tundra.
However, strong heterogeneity in responses to the experimental
warming suggested that other factors could moderate the effects of
climate warming significantly, such as herbivory, differences in soil
nutrients and pH, precipitation, winter temperatures and snow cover,
and species composition and density.
Snow bed habitats have decreased in sub-Arctic Sweden (Björk and Molau,
2007; Hedenås et al., 2011). In other plant communities, changes have
been less dramatic, ranging from small increases in species richness in the
south west Yukon of the Canadian sub-Arctic (Danby et al., 2011), through
subtle changes in plant community composition in west and southeast
Greenland (Callaghan et al., 2011a; Daniëls and De Molenaar, 2011) to
70-year stability of a plant community on Svalbard (Prach et al., 2010).
>2 10–1 –2 –2.9 –3.9 –4.8 –5.7 –6.5 <–7.4
<–2 –1 01234 567>8
Trend in seasonality with respect to 1982 (% per decade)
Trend in PAP mean NDVI with respect to 1982 (% per decade)
1
20°E
1
50°E
180°E
1
50°W
120°W
90°W
0°
9
0°E
6
0°E
60°W
3
0°E
3
0°W
9
0°N
75°N
65°N
55°N
45°N
Figure 28-3 | Significant changes (p < 0.01) in photosynthetically active period (PAP) Normalized Difference Vegetation Index (NDVI) between 1982 and 2012 (Xu et al., 2013).

1580
Chapter 28 Polar Regions
28
T
he responses to Arctic warming of lichen and bryophyte (mosses)
diversity have been heterogeneous, varying from consistent negative
effects to significant increases in recent years (Hudson and Henry, 2009;
Tømmervik et al., 2009, 2012). Forbes and Kumpula (2009) recorded long-
term and widespread lichen degradation in northern Finland attributed
more to trampling of dry lichens by reindeer in summer than to winter
consumption as forage.
Palaeorecords of vegetation change indicate that the northern tree line
should extend upward and northward during current climate warming
(Callaghan et al., 2005) because tree line is related to summer warmth
(e.g., Harsch et al., 2009). Although the tree line has moved northward
and upward in many Arctic areas, it has not shown a general circumpolar
expansion in recent decades (high confidence).
Model projections that suggest a displacement of between 11 and 50%
of tundra by forest by 2100 (see references in Callaghan et al., 2005)
and shifts upslope by 2 to 6 m yr
–1
(Moen et al., 2004) and northwards
by 7.4 to 20 km yr
–1
(Kaplan and New, 2006) might be overestimating
rate of tree line advance by a factor of up to 2000 (Van Bogaert et al.,
2011). The fastest upslope shifts of tree lines recorded during 20th century
warming are 1 to 2 m yr
–
1
(Shiyatov et al., 2007; Kullman and Öberg, 2009)
whereas the fastest so-far recorded northward-migrating tree line replaces
tundra by taiga at a rate of 3 to 10 m yr
–
1
(Kharuk et al., 2006). In some
areas, the location of the tree line has not changed or has changed very
slowly (Payette, 2007; MacDonald et al., 2008). A global study by Harsch
et al. (2009) showed that only 52% of 166 global tree line sites studied
had advanced over the past 100 years. In many cases the tree line has
even retreated (Cherosov et al., 2010). At the small scale, the tree line
has shown increase, decrease, and stability in neighboring locations
(Lloyd et al., 2011; Van Bogaert et al., 2011).
Evidence for densification of the forest at the sub-Arctic tree line is
robust and consistent within Fennoscandia (Tømmervik et al., 2009;
Hedenås et al., 2011; Rundqvist et al., 2011) and Canada (Danby and Hik,
2007). Dendroecological studies indicate enhanced conifer recruitment
during the 20th century in the northern Siberian taiga (Briffa et al.,
2008). Some of the changes are dramatic, such as an increase in area
of mountain birch in study plots in northern Sweden by 600% between
1977/1998 and 2009/2010 (Rundqvist et al., 2011) and a doubling of
tree biomass in Finnmarksvidda in northern Norway since 1957
(Tømmervik et al., 2009). However, model projections of displacement
of deciduous forest by evergreen forest (Wolf et al., 2008; Wramneby
et al., 2010) have not so far been validated.
Where the mountain birch tree line has increased in elevation and shrub
(e.g., willow, dwarf birch) abundance has increased, the response can
be an interaction between climate warming, herbivory pressure, and
earlier land use (Olofsson et al., 2009; Hofgaard et al., 2010; Van Bogaert
et al., 2011). In Fennoscandia and Greenland, heavy grazing by large
herbivores may significantly check deciduous low erect shrub (e.g.,
dwarf shrub and willow) growth (Post et al., 2008; Kitti et al., 2009;
Olofsson et al., 2009).
Less moisture from snow and more rain now favors broadleaf trees over
conifers and mosses in some areas (Juday, 2009) while moisture deficits
are reducing the growth of some northern forests (Goetz et al., 2005;
V
erbyla, 2008; Yarie, 2008) and making them more susceptible to insect
pest outbreaks (see references in Callaghan et al., 2011c). Death of
trees through drought stress or insect pest activity will increase the
probability of fire, which will have positive feedbacks (increase warming)
on the climate (Mack et al., 2011).
2
8.2.3.1.3. Changes in animal populations
The documented collapse or dampening of population cycles of voles
and lemmings over the last 20 to 30 years in parts of Fennoscandia and
Greenland (Schmidt et al., 2012) can be attributed with high confidence
to climate change (Ims et al., 2007, 2011; Gilg et al., 2009; Kausrud et
al., 2009). A shortening of the snow season and more thaw and/or rain
events during the winter will have an effect on the subnivean space,
which provides thermal insulation, access to food, and protection from
predators (Berg et al., 2008; Kausrud et al., 2009; Johansson et al., 2011).
However, the causes of the changes in the lemming and vole cycles are
still being debated as factors other than climate change may also be of
importance (Brommer et al., 2010; Krebs, 2011).
Climate-mediated range expansion both in altitude and latitude of insect
pests, and increased survival due to higher winter temperatures, has been
documented for bark beetles in North America (Robertson et al., 2009)
and for geometrid moths in Fennoscandia (Jepsen et al., 2008, 2011;
Callaghan et al., 2010), causing more extensive forest damage than
before. Outbreaks of insect pests such as geometrid moths can even
reduce the strengths of CO
2
sinks in some areas (Heliasz et al., 2011).
The decline in wild reindeer and caribou (both Rangifer tarandus)
populations in some regions of about 30% over the last 10 to 15 years
has been linked both to climate warming and anthropogenic landscape
changes (Post et al., 2009a; Vors and Boyce, 2009; Russell and Gunn,
2010). Even though most of the Arctic has warmed, the decline in the
populations has not been uniform. Some of the North American large,
wild herds have, for example, declined by 75 to 90%, while other wild
herds and semi-domestic herds in Fennoscandia and Russia have been
stable or even increased (Forbes et al., 2009; Gunn et al., 2009; Vors
and Boyce, 2009; Forbes, 2010; Joly et al., 2011; Kumpula et al., 2012).
The expected and partially observed increased primary productivity of
Arctic tundra may potentially increase the supply of food for Arctic
ungulates. However, the overall quality of forage may decline during
warming, for example, if the nitrogen content of key fodder species for
ungulates were to drop during warming (Turunen et al., 2009;
Heggberget et al., 2010), while lichen biomass, an important winter fodder
for reindeer, is decreasing over parts of the Arctic region. Herbivory also
changes the vegetation itself in concert with the warming, further
complicating the prediction of vegetation changes and their impacts on
ungulate populations (van Der Wal et al., 2007; Turunen et al., 2009).
More frequent rain-on-snow icing events and thicker snowpacks caused
by warmer winters and increased precipitation may restrict access to
vegetation and may have profound negative influences on the population
dynamics of Arctic ungulates (Berg et al., 2008; Forchhammer et al.,
2008; Miller and Barry, 2009; Stien et al., 2010, 2012; Hansen et al.,
2011). Such events have caused heavy mortality in some semi-domestic

1581
Polar Regions Chapter 28
28
r
eindeer herds and musk oxen in recent years (Grenfell and Putkonen,
2008; Forbes, 2009; Bartsch et al., 2010), and have also been shown to
synchronize the dynamics of a resident vertebrate community (small
mammals, reindeer, and Arctic fox) in Svalbard (Hansen et al., 2013). In
contrast, Tyler et al. (2008) and Tyler (2010) suggested that generally
warmer winters enhance the abundance of reindeer populations.
It has been suggested that warming-induced trophic mismatches
between forage availability and quality and timing of calving have a
role in the decline of circumpolar reindeer and caribou populations (Post
and Forchhammer, 2008; Post et al., 2009a,b), although such trophic
mismatch has been disputed (Griffith et al., 2010).
Adjustment via phenotypic plasticity instead of adaptation by natural
selection is expected to dominate vertebrate responses to rapid
Arctic climate change, and many such adjustments have already been
documented (Gilg et al., 2012).
28.2.3.1.4. Long-term trends and event-driven changes
Long-term climate change impacts on vegetation and animal populations
are accelerated when tipping points are triggered by events such as
extreme weather, fire, insect pest, and disease outbreaks. The impacts
of winter thaw events on ecosystems are now well documented (e.g.,
Bokhorst et al., 2011) but studies of the severe impacts of tundra fires
on vegetation and biospheric feedbacks are recent (Mack et al., 2011).
Results from experimental winter thaws were validated by a natural
event in northern Norway and Sweden in 2007 that reduced NDVI by
almost 30% over at least 1400 km
2
(Bokhorst et al., 2009). Studies on
relationships between climate change and plant disease are rare, but
Olofsson et al. (2011) showed that increased snow accumulation led to
a higher incidence of fungal growth on sub-Arctic vegetation.
28.2.3.2. Antarctica
Antarctic terrestrial ecosystems occur in 15 biologically distinct areas
(Terauds et al., 2012), with those in the maritime and sub-Antarctic
islands experiencing the warmest temperatures, reduced extreme
seasonality and greatest biodiversity (Convey, 2006). In the cooler
conditions on the continent, species must be capable of exploiting the
short periods where temperature and moisture availability are above
physiological and biochemical thresholds. In many areas, there is no
visible vegetation, with life being limited, at the extreme, to endolithic
(within rock) communities of algae, cyanobacteria, fungi, bacteria, and
lichens (Convey, 2006).
Few robust studies are available of biological responses to observed
climatic changes in natural Antarctic terrestrial ecosystems. The rapid
population expansion and local-scale colonization by two native
flowering plants (Deschampsia antarctica and Colobanthus quitensis) in
maritime Antarctica (Parnikoza et al., 2009) remains the only published
repeat long-term monitoring study of any terrestrial vegetation or
location in Antarctica. Radiocarbon dating of moss peat deposits has
shown that growth rates and microbial productivity have risen rapidly
on the Antarctic Peninsula since the 1960s, consistent with temperature
c
hanges, and are unprecedented in the last 150 years (Royles et al.,
2013). In east Antarctica, moss growth rates over the last 50 years
have been linked to changes in wind speed and temperature and their
influence on water availability (Clarke et al., 2012). A contributing factor
is that air temperatures have increased past the critical temperature at
which successful sexual reproduction (seed set) can now take place,
changing the dominant mode of reproduction and increasing the
potential distance for dispersal (low confidence; Convey, 2011). Similar
changes in the local distribution and development of typical cryptogamic
vegetation of this region have been reported (Convey, 2011), including
the rapid colonization of ice-free ground made available through glacial
retreat and reduction in extent of previously permanent snow cover
(Olech and Chwedorzewska, 2011). As these vegetation changes create
new habitat, there are concurrent changes in the local distribution and
abundance of the invertebrate fauna that then colonize them (low
confidence).
28.2.4. Health and Well-being of Arctic Residents
The warming Arctic and major changes in the cryosphere are significantly
impacting the health and well-being of Arctic residents and projected
to increase, especially for many Indigenous peoples. Although impacts
are expected to vary among the diverse settlements that range from
small, remote, predominantly Indigenous to large cities and industrial
settlements, this section focuses more on health impacts of climate
change on Indigenous, isolated, and rural populations because they are
especially vulnerable to climate change owing to a strong dependence
on the environment for food, culture, and way of life; their political and
economic marginalization; existing social, health, and poverty disparities;
as well as their frequent close proximity to exposed locations along
ocean, lake, or river shorelines (Ford and Furgal, 2009; Galloway-McLean,
2010; Larsen et al., 2010; Cochran et al., 2013).
28.2.4.1. Direct Impacts of a Changing Climate
on the Health of Arctic Residents
Direct impacts of climate changes on the health of Arctic residents
include extreme weather events, rapidly changing weather conditions,
and increasingly unsafe hunting conditions (physical/mental injuries,
death, disease), temperature-related stress (limits of human survival in
thermal environment, cold injuries, cold-related diseases), and UV-B
radiation (immunosuppression, skin cancer, non-Hodgkin’s lymphoma,
cataracts) (high confidence; Revich, 2008; AMAP, 2009; IPCC, 2012).
Intense precipitation events and rapid snowmelt are expected to impact
the magnitude and frequency of slumping and active layer detachment,
resulting in rock falls, debris flow, and avalanches (Kokelj et al., 2009;
Ford et al., 2010). Other impacts from weather, extreme events, and
natural disasters are the possibility of increasingly unpredictable, long
duration, and/or rapid onset of extreme weather events, storms, and
inundation by large storm surges, which, in turn, may create risks to safe
travel or subsistence activities, loss of access to critical supplies and
services to rural or isolated communities (e.g., food, telecommunications,
fuel), and risk of being trapped outside one’s own community (high
confidence; Laidre et al., 2008; Parkinson, 2009; Brubaker et al., 2011b,c).
Changing river and sea ice conditions affect the safety of travel for

1582
Chapter 28 Polar Regions
28
I
ndigenous populations especially, and inhibit access to critical hunting,
herding, and fishing areas (Andrachuk and Pearce, 2010; Derksen et al.,
2012; Huntington and Watson, 2012).
Cold exposure has been shown to increase the frequency of certain
injuries (e.g., hypothermia, frostbite), accidents, and diseases (respiratory,
circulatory, cardiovascular, musculoskeletal) (Revich and Shaposhmikov,
2010). Studies in northern Russia have indicated an association between
low temperatures and social stress and cases of cardiomyopathy (Revich
and Shaposhnikov, 2010). It is expected that winter warming in the
Arctic will reduce winter mortality rates, primarily through a reduction
in respiratory and cardiovascular deaths (Shaposhnikov et al., 2010).
Researchers project that a reduction in cold-related injuries may occur,
assuming that the standard for protection against the cold is not
reduced (including individual behavior-related factors) (Nayha, 2005).
Conversely, studies are showing respiratory and cardiac stress associated
with extreme warm summer days and that rising temperatures are
accompanied by increased air pollution and mortality, especially in
Russian cities with large pollution sources (Revich, 2008; Revich and
Shaposhnikov, 2012).
28.2.4.2. Indirect Impacts of Climate Change
on the Health of Arctic Residents
Indirect effects of climate change on the health of Arctic residents
include a complex set of impacts such as changes in animal and
plant populations (species responses, infectious diseases), changes in
the physical environment (ice and snow, permafrost), diet (food yields,
availability of country food), built environment (sanitation infrastructure,
water supply system, waste systems, building structures), drinking water
access, contaminants (local, long-range transported), and coastal issues
(harmful algal blooms, erosion) (high confidence; Maynard and Conway,
2007; Parkinson and Evengård, 2009; Brubaker et al., 2011a; see also
Chapter 11).
In addition to the climate change impacts and processes are the
complicated impacts from contaminants such as persistent organic
pollutants (POPs), radioactivity, and heavy metals (e.g., mercury), which
create additional and/or synergistic impacts on the overall health and
well-being of all Arctic communities (Armitage et al., 2011; UNEP and
AMAP, 2011; Teran et al., 2012). Ambient temperature variability and
temperature gradients directly affect the volatilization, remobilization,
and transport pathways of mercury and POPs in the atmosphere, ocean
currents, sea ice, and rivers. Transport pathways, inter-compartmental
distribution, and bioaccumulation and transformation of environmental
contaminants such as POPs, mercury, and radionuclides in the Arctic
may consequently be affected by climate change (high confidence;
AMAP 2011b; Ma et al., 2011; UNEP and AMAP 2011; Teng et al., 2012).
Ma et al. (2011) and Hung et al. (2010) demonstrated that POPs are
already being remobilized into the air from sinks in the Arctic region as
a result of decreasing sea ice and increasing temperatures.
Contaminants and human health in the Arctic are tightly linked to the
climate and Arctic ecosystems by factors such as contaminant cycling
and climate (increased transport to and from the Arctic), and the related
increased risks of transmission to residents through subsistence life
w
ays (Maynard, 2006; AMAP, 2010; Armitage et al., 2011; UNEP and
AMAP, 2011; Teran et al., 2012). The consumption of traditional foods
by Indigenous peoples places these populations at the top of the Arctic
food chain and through biomagnification, therefore, they may receive
some of the highest exposures in the world to certain contaminants
(Armitage et al., 2011; UNEP and AMAP, 2011). Contaminants such as
POPs are known for their adverse neurological and medical effects
on humans, particularly the developing fetus, children, women of
reproductive age, and the elderly; thus it is important to include
contaminants as a significant part of any climate impact assessment
(UNEP and AMAP, 2011).
Radioactivity in the Arctic is also a concern because there are many
potential and existing radionuclide sources in some parts of the Arctic,
and contamination can remain for long periods of time in soils and some
vegetation, creating potentially high exposures for people (AMAP, 2010).
Climate changes can mobilize radionuclides throughout the Arctic
environment, and also potentially impact infrastructure associated with
nuclear activities by changes in permafrost, precipitation, erosion, and
extreme weather events (AMAP, 2010).
Warming temperatures are enabling increased overwintering survival
and distribution of new insects that sting and bite as well as many bird,
animal, and insect species that can serve as disease vectors and, in turn,
causing an increase in human exposure to new and emerging infectious
diseases (Parkinson et al., 2008; Epstein and Ferber, 2011). Examples of
new and emerging diseases are tick-borne encephalitis (brain infection)
in Russia and Canada (Ogden et al., 2010; Tokarevich et al., 2011)
and Sweden (Lindgren and Gustafson, 2001) and Giardia spp. and
Cryptosporidium spp. infection of ringed seals (Phoca hispida) and
bowhead whales (Balaena mysticetus) in the Arctic Ocean (Hughes-
Hanks et al., 2005). It is also expected that temperature increases will
increase the incidence of zoonotic diseases as relocations of animal
populations occur (Revich et al., 2012; Hueffler et al., 2013).
Harmful algal blooms (HABs), whose biotoxins can be a serious health
hazard to humans or animals (paralysis, death), are increasing globally
and expected to increase in the Arctic, and HABs are influenced directly
by climate change-related factors such as temperature, winds, currents,
nutrients, and runoff (Portier et al., 2010; Epstein and Ferber, 2011; Walsh
et al., 2011; see also Chapters 6, 11). Increasing ocean temperatures
have caused an outbreak of a cholera-like disease, caused by Vibrio
parahaemolyticus, in Alaskan oysters (McLaughlin et al., 2005). In
addition, warmer temperatures raise the possibility of anthrax exposure
in Siberia from permafrost thawing of historic cattle burial grounds
(Revich and Podolnaya, 2011).
The impacts of climate change on food security and basic nutrition are
critical to human health because subsistence foods from the local
environment provide Arctic residents, especially Indigenous peoples,
with unique cultural and economic benefits necessary to well-being and
contribute a significant proportion of daily requirements of nutrition,
vitamins, and essential elements to the diet (Ford, 2009; Ford and
Berrang-Ford, 2009). However, climate change is already an important
threat because of the decrease in predictability of weather patterns, low
water levels and streams, timing of snow, and ice extent and stability,
impacting the opportunities for successful hunting, gathering, fishing,

1583
Polar Regions Chapter 28
28
a
nd access to food sources and increasing the probability of accidents
(high confidence; Ford and Furgal, 2009; Ford et al., 2010). In recent years,
populations of marine and land mammals, fish, and water fowl are also
being reduced or displaced, thus reducing the traditional food supply
(Gearheard et al., 2006; West and Hovelsrud, 2010; Lynn et al., 2013).
Traditional food preservation methods such as drying of fish and meat,
fermentation, and ice cellar storage are being compromised by warming
temperatures, thus further reducing food available to the community
(Brubaker et al., 2011b,c). For example, food contamination caused by
thawing of permafrost “ice cellars” is occurring and increasingly wet
conditions make it harder to dry food for storage (Hovelsrud et al.,
2011). Indigenous people increasingly have to abandon their semi-
nomadic lifestyles, limiting their overall flexibility to access traditional
foods from more distant locations (www.arctichealthyukon.ca). These
reductions in the availability of traditional foods plus general globalization
pressures are forcing Indigenous communities to increasingly depend
on expensive, non-traditional, and often less healthy Western foods,
increasing the rates of modern diseases associated with processed food
and its packaging, such as cardiovascular diseases, diabetes, dental
caries, and obesity (Armitage et al., 2011; Berrang-Ford et al., 2011;
Brubaker et al., 2011b,c).
Climate change is beginning to threaten community and public health
infrastructure, often in communities with no central water supply and
treatment sources. This is especially serious in low-lying coastal Arctic
communities (e.g., Shishmaref, Alaska, USA; Tuktoyaktuk, Northwest
Territories, Canada) through increased river and coastal flooding and
erosion, increased drought, and thawing of permafrost, resulting in loss
of reservoirs, damage to landfill sites, or sewage contamination (GAO,
2009; Bronen, 2011). Saltwater intrusion and bacterial contamination
may also be threatening community water supplies (Parkinson et al.,
2008; Virginia and Yalowitz, 2012). Quantities of water available for
drinking, basic hygiene, and cooking are becoming limited owing to
damaged infrastructure, drought, and changes in hydrology (Virginia
and Yalowitz, 2012). Disease incidence caused by contact with human
waste may increase when flooding and damaged infrastructure spreads
sewage in villages with no municipal water supply. This can result in
higher rates of hospitalization for pneumonia, influenza, skin infections,
and respiratory viral infections (Parkinson and Evengård, 2009; Virginia
and Yalowitz, 2012). Compounding these impacts in rural areas as well
as cities are respiratory and other illnesses caused by air-borne pollutants
(e.g., contaminants, microbes, dust, mold, pollen, smoke) (Revich, 2008;
Rylander and Schilling, 2011; Revich and Shaposhnikov, 2012).
It is now well documented that the many climate-related impacts on
Arctic communities are causing significant psychological and mental
distress and anxiety among residents (Levintova, 2010; Portier et al.,
2010; Coyle and Susteren, 2012; see also Chapter 11). For example,
changes in the physical environment (e.g., through thawing permafrost
and erosion) that may lead to forced or voluntary relocation of residents
out of their villages or loss of traditional subsistence species are causing
mental health impacts among Indigenous and other vulnerable, isolated
populations (Curtis et al., 2005; Albrecht et al., 2007; Coyle and Susteren,
2012; Maldonado et al, 2013). Special concern has been expressed by
many communities about the unusually high and increasing numbers
of suicides in the Arctic, especially among Indigenous youth, and efforts
a
re underway to try to develop a thorough assessment as well as
establish effective intervention efforts (Albrecht et al., 2007; Portier et
al., 2010; USARC, 2010).
28.2.5. Indigenous Peoples and Traditional Knowledge
Indigenous populations in the Arctic—the original Native inhabitants
of the region—are considered especially vulnerable to climate change
because of their close relationship with the environment and its natural
resources for physical, social, and cultural well-being (Nuttall et al.,
2005; Parkinson, 2009; Cochran et al., 2013). Although there are wide
differences in the estimates, including variations in definitions of the
Arctic region, Arctic Indigenous peoples are estimated to number
between 400,000 and 1.3 million (Bogoyavlensky and Siggner, 2004;
Galloway-McLean, 2010). According to 2010 census data, there are
approximately 68,000 Indigenous people living in the Russian Arctic.
These Arctic residents depend heavily on the region’s terrestrial, marine,
and freshwater renewable resources, including fish, mammals, birds,
and plants; however, the ability of Indigenous peoples to maintain
traditional livelihoods such as hunting, harvesting, and herding is
increasingly being threatened by the unprecedented rate of climate
change (high confidence; Nakashima et al., 2012; Cochran et al., 2013). In
habitats across the Arctic, climate changes are affecting these livelihoods
through decreased sea ice thickness and extent, less predictable weather,
severe storms, sea level rise, changing seasonal melt/freeze-up of rivers
and lakes, changes in snow type and timing, increasing shrub growth,
permafrost thaw, and storm-related erosion, which, in turn, are causing
such severe loss of land in some regions that a number of Alaskan
coastal villages are having to relocate entire communities (Oskal, 2008;
Forbes and Stammler, 2009; Mahoney et al., 2009; Bartsch et al., 2010;
Weatherhead et al., 2010; ,Bronen, 2011; Brubaker et al., 2011b,c; Eira et
al., 2012; Huntington and Watson, 2012; McNeeley, 2012; Maldonado et
al., 2013). In addressing these climate impacts, Indigenous communities
must at the same time consider multiple other stressors such as resource
development (oil and gas, mining); pollution; changes in land use policies;
changing forms of governance; and the prevalence in many Indigenous
communities of poverty, marginalization, and resulting health disparities
(Abryutina, 2009; Forbes et al., 2009; Reinert et al., 2009; Magga et al.,
2011; Vuojala-Magga et al., 2011; Nakashima et al., 2012; Mathiesen
et al., 2013).
Traditional knowledge is the historical knowledge of Indigenous peoples
accumulated over many generations and it is increasingly emerging as
an important knowledge base for more comprehensively addressing the
impacts of environmental and other changes as well as development of
appropriate adaptation strategies for Indigenous communities (WGII AR4
Chapter 15; Oskal, 2008; Reinert et al., 2008; Wildcat, 2009; Magga et
al., 2011; Vuojala-Magga et al., 2011; Nakashima et al., 2012; Vogesser
et al, 2013). For example, Saami reindeer herders have specialized
knowledge of dynamic snow conditions, which mediate access to forage
on autumn, winter, and spring reindeer rangelands (Roturier and Roue,
2009; Eira et al., 2012; Vikhamar-Schuler et al., 2013) and traditional
governance systems for relating to natural environments (Sara, 2013).
Increasingly, traditional knowledge is being combined with Western
scientific knowledge to develop more sustainable adaptation strategies
for all communities in the changing climate.

1584
Chapter 28 Polar Regions
28
F
or example, at Clyde River, Nunavut, Canada, Inuit experts and scientists
both note that wind speed has increased in recent years and that wind
direction changes more often over shorter periods (within a day) than
it did during the past few decades (Gearheard et al., 2010; Overland et
al., 2012). In Norway, Sámi reindeer herders and scientists are both
observing direct and indirect impacts to reindeer husbandry such as
changes in snow and ice cover, forage availability, and timing of river
freeze-thaw patterns from increasing temperatures (Eira et al., 2012).
On the Yamal Peninsula in western Siberia, detailed Nenets observations
and recollections of iced-over autumn and winter pastures due to rain-
on-snow events have proven suitable for calibrating the satellite-based
microwave sensor SeaWinds (Bartsch et al., 2010) and NASA’s AMSR-E
sensor.
28.2.6. Economic Sectors
28.2.6.1. Arctic
28.2.6.1.1. Agriculture and forestry
Climate change presents benefits and costs for forestry and agriculture
(Aaheim et al., 2009; Hovelsrud et al., 2011). In Iceland, for example, tree
limits are found at higher altitudes than before, and productivity of many
plants has increased (Björnsson et al., 2011). Grain production in Iceland
has increased in the last 2 decades, and work on soil conservation and
forestry has benefited from warming (Sigurdsson et al., 2007; Björnsson
et al., 2011), but also the number of new insect pests on trees and shrubs
has increased in the past 20 years. A strong relationship between rate
of new insect pest colonization and outbreak intensity in forests exists
with changes in annual temperature during the past century (Halldórsson
et al., 2013). Climate change impacts on species change and fire
frequency have potential impact on commercial forest harvesting activity.
Vulnerability of forestry to changes that affect road conditions and thus
accessibility during thawing periods has been found in Sweden (Keskitalo,
2008). A case study on Greenland found challenges for plant diseases
in potatoes and grass fields, with pathogens and pests present in
agricultural cropping systems, for example, black scurf (Rhizoctonia)
and common scab (Streptomyces scabies) (Neergaard et al., 2009).
28.2.6.1.2. Open and freshwater fisheries
Current commercial fisheries are sharply divided between regions of
high-yield and value (e.g., commercial fisheries in the southern Bering
Sea, Baffin Bay, the east and west Greenland Seas, the Iceland Shelf
Sea, the deep Norwegian/Greenland Sea, and the Barents Sea) and
subsistence fisheries in the coastal regions of the Arctic Ocean. The
relative absence of commercial fishing activity in the Arctic Ocean results
from a combination of fisheries policy, the abundance of the resource,
the lack of infrastructure for capturing and processing fish, and the
difficulties in accessing fishing grounds, especially during winter. In most
regions, fisheries management strategies have been developed to build
sustainable fisheries and rebuild overfished stocks (Froese and Proelß,
2010; Livingston et al., 2011). Recently observed changes in the spatial
distribution and abundance of mackerel (Scomber scombrus) has
challenged existing international agreements for shared resources in
t
he North Atlantic (Arnason, 2012; Astthorsson et al., 2012). Although
loss of sea ice in summer is allowing greater access to fisheries
resources in the Arctic Ocean, some nations have prohibited commercial
fishing within their exclusive economic zones until there is sufficient
understanding of stock status to ensure that proposed fisheries would
be managed sustainably (Stram and Evans, 2009; Wilson and Ormseth,
2009).
Several Arctic coastal sea-run fishes are targeted for subsistence and
commercial use in the Arctic. Commercial transactions from fishing are
typically for local markets; however, the socioeconomic and cultural
importance of these fishes to Indigenous peoples far outweighs their
monetary value. Reist et al. (2006) and Fechhelm et al. (2007) found that
climate-related factors that influenced the water level and freshening of
rivers were related to run size of Arctic cisco (Coregonus autumnalis).
Similarly, a recent study based on Chinook salmon (Oncorhynchus
tshawytscha) run timing for the period 1961–2009 showed that success
in the fishery was dependent on the timing of the marine exit, which
was tightly coupled to environmental conditions that were linked to
climate (Mundy and Evenson, 2011).
28.2.6.1.3. Marine transportation
Observations and climate models indicate that in the period between
1979–1988 and 1998–2007 the number of days with ice-free conditions
(less than 15% ice concentration) increased by 22 days along the
Northern Sea Route (NSR) in the Russian Arctic, and by 19 days in the
Northwest Passage (NWP) in the Canadian Arctic, while the average
duration of the navigation season in the period 1980–1999 was 45 and
35 days, respectively (Mokhow and Khon, 2008). Increased shipping
associated with the opening of the NSR will lead to increased resource
extraction on land and in the sea, and with two-way commodity flows
between the Atlantic and Pacific. The future status of marine, terrestrial,
and freshwater biota may be negatively affected as a result of substantial
coastal infrastructure to facilitate offshore developments (Meschtyb et
al., 2005). Also, the frequency of marine transportation along the NSR
is at its highest during the most productive and vulnerable season for
fish and marine mammals, which is the late spring/summer, when these
resources can be found throughout the NSR area (Østreng, 2006).
28.2.6.1.4. Infrastructure
Much of the physical infrastructure in the Arctic relies on and is adapted
to local sea ice conditions, permafrost, and snow (Huntington et al., 2007;
Sundby and Nakken, 2008; Sherman et al., 2009; West and Hovelsrud,
2010; Forbes, 2011). Damage from ice action and flooding to installations
such as bridges, pipelines, drilling platforms, and hydropower poses
major economic costs and risks, which are more closely linked to the design
of the structure than with thawing permafrost. Current engineering
practices are designed to help minimize the impacts (Prowse et al., 2009).
Much of the infrastructure has been built with weather conditions in mind,
but remains vulnerable and inadequate to respond to environmental
emergencies, natural disasters, and non-environmental accidents (NRTEE,
2009). Northern safety, security, and environmental integrity are much
dependent on transportation infrastructure. Ice as a provisioning system

1585
Polar Regions Chapter 28
28
p
rovides a transportation corridor and a platform for a range of activities
and access to food sources in the Arctic (Eicken et al., 2009).
In northern Canada climate warming presents an additional challenge
for northern development and infrastructure design. While the impacts
of climate change become increasingly significant over the longer time
scales, in the short term of greater significance will be the impacts
associated with ground disturbance and construction (Smith and
Risebrough, 2010).
Climate change impacts have increased the demand for improved
communication infrastructure and related services and community
infrastructure for the safety and confidence in drinking water (NRTEE,
2009). The access, treatment, and distribution of drinking water is
generally dependent on a stable platform of permafrost for pond or lake
retention. Several communities have reported the need for more
frequent water-quality testing of both municipal systems and untreated
water sources to ensure its suitability for drinking (Furgal, 2008).
28.2.6.1.5. Resource exploration
The Arctic has large reserves of minerals (Lindholt, 2006; Harsem et al.,
2011; Peters et al., 2011) and potentially large reserves of undiscovered
sources of raw minerals and oil and gas. Predicted new access to
offshore energy resources is hypothesized to be a significant share of
the global supply of oil and gas (Gautier et al., 2009; Berkman, 2010).
The socioeconomic impacts of oil and gas exploration activity may be
positive or negative (Duhaime et al., 2004; Huntington et al., 2007; Forbes,
2008; Forbes et al., 2009; Kumpula et al., 2011; Harsem et al., 2011).
Climatic warming is accelerating access to northern lands for development
(Forbes et al., 2009). Yamal in Western Siberia has approximately 90%
of Russia’s gas reserves, but at the same time represents the largest
area of reindeer herding in the world (Jernsletten and Klokov, 2002;
Stammler, 2005; Forbes and Kumpula, 2009). Development activities to
obtain these resources would shrink the grazing lands, and have
been characterized as one of the major human activities in the Arctic
contributing to loss of “available room for adaptation” for reindeer
husbandry (Nuttall et al., 2005; Oskal, 2008; Forbes et al., 2009). Sharp
increases in future oil and gas and other resource development in the
Russian north and other Arctic regions are anticipated—along with
associated infrastructure, pollution, and other development byproducts—
which will reduce the availability of pasturelands for reindeer and use
by Indigenous communities (Derome and Lukina, 2011; Degteva and
Nellermann, 2013).
28.2.6.1.6. Informal, subsistence-based economy
Hunting, gathering, herding, and fishing for subsistence, as well as
commercial fishing, all play an important role in the mixed cash-
subsistence economies (Nuttall et al., 2005; Poppel and Kruse, 2009; Crate
et al., 2010; Larsen and Huskey, 2010). In the early 1990s—initially in
western Canada, and later elsewhere—Indigenous communities started
reporting climate change impacts (Berkes and Armitage, 2010). According
to some herders, whalers, and walrus hunters, non-predictable conditions
r
esulting from more frequent occurrence of unusual weather events are
the main effect of recent warming (Forbes and Stammler, 2009; Forbes
et al., 2009; Ignatowski and Rosales, 2013).
The Inuit and Saami have expressed strong concern about the effects
of climate warming on their livelihoods (Forbes and Stammler, 2009;
Magga et al., 2011). For the Inuit, the issues revolve around sea ice
conditions, such as later freeze-up in autumn; earlier melt-out and faster
sea ice retreat in spring; and thinner, less predictable ice in general
(Krupnik and Jolly, 2002; Cochran et al., 2013). Diminished sea ice
translates into more difficult access for hunting marine mammals, and
greater risk for the long-term viability of subsistence species such as
polar bear populations (high confidence; Laidre et al., 2008). Most Inuit
communities depend to some extent on marine mammals for nutritional
and cultural reasons, and many benefit economically from polar bear
and narwhal hunting. A reduction in these resources represents a
potentially significant economic loss (Hovelsrud et al., 2008). Among
Fennoscandian Saami, the economic viability of reindeer herding is
threatened by competition with other land users coupled with strict
agricultural norms (Forbes, 2006; Magga et al., 2011). Reindeer herders
are concerned that more extreme weather may exacerbate this situation
(Oskal, 2008).
Climate change is affecting reindeer herding communities through greater
variability in snow melt/freeze, ice, weather, winds, temperatures, and
precipitation, which, in turn are affecting snow quality and quantity—
the most critical environmental variables for reindeer sustainability
(Bartsch et al., 2010; Magga et al., 2011; Eira et al., 2012). Increasing
temperature variations in wintertime, with temperatures rising above
freezing with rain, followed by refreezing (“rain-on-snow” conditions),
are becoming more frequent, forming ice layers in the snow that then
block the animals’ access to their forage and subsequent starvation
(Bartsch, 2010; Maynard et al., 2011; Eira et al., 2012).
28.2.6.2. Antarctica and the Southern Ocean
Economic activities in the Antarctic have been limited to fishing and
tourism (IPCC, 2007). Ship-based tourism is a significant industry in
Antarctica but does not involve permanent shore-based infrastructure.
Over recent decades, the number of tourists landing in Antarctica has
risen from 7322 in 1996/1997 to 32,637 in 2007/2008 (IAATO, 2012).
Visits generally coincide with the times when wildlife are breeding and
are often restricted because of the presence of fast ice, sea ice, or icebergs.
They are expected to continue to increase, with an increasing chance
of terrestrial alien species being introduced from tourism and other
vectors as ice-free areas increase from climate change (Chown et al.,
2012). Scientific activity by a number of nations is also taking place and
has the potential to impact upon local ecologies. Mineral resource
activity is prohibited south of 60°S under the Protocol on Environmental
Protection to the Antarctic Treaty.
Fisheries in Antarctica, primarily through fisheries for Antarctic krill, could
amount to approximately 6% of existing global marine capture fisheries
(Nicol et al., 2012). The pattern of the krill fishery has been affected by
changes in the sea ice extent around the Antarctic Peninsula, where the
fishery has been taking advantage of the ice-free conditions and taking

1586
Chapter 28 Polar Regions
28
m
ore of its catch during winter in that region (high confidence; Kawaguchi
et al., 2009). Ecosystem-based management of krill fisheries by the
Commission for the Conservation of Antarctic Marine Living Resources
(CCAMLR) has yet to include procedures to account for climate change
impacts, although the need to do so has been identified (Trathan and
Agnew, 2010; Constable, 2011).
28.3. Key Projected Impacts and Vulnerabilities
28.3.1. Hydrology and Freshwater Ecosystems
28.3.1.1. Arctic
Accompanying projected increases in high-latitude river flow (Section
3.4.5; WGI AR5 Section 12.4.5.4) are earlier spring runoff (Pohl et al.,
2007; Dankers and Middelkoop, 2008; Hay and McCabe, 2010), greater
spring snowmelt (Adam et al., 2009), and increases in spring sediment
fluxes (Lewis and Lamoureux, 2010). Enhanced permafrost thaw (WGI
AR5 Section 12.4.6.2) will continue to affect the dynamics of thermokarst
lakes and related ecological effects (Section 28.2.1.1). Thawing permafrost
and changes in the hydrological regime of the Arctic rivers, particularly
those traversing regions affected by industrial developments, will increase
the contaminant flow (Nikanorovet al.,2007). Loss of glacier ice masses
will alter runoff hydrographs; sediment loads; water chemistry; thermal
regimes; and related channel stability, habitat, and biodiversity (Milner
et al., 2009; Moore et al., 2009). Although snow, freshwater ice, and
permafrost affect the morphology of arctic alluvial channels, their future
combined effects remain unclear (McNamara and Kane, 2009). For small
permafrost streams, however, longer projected periods of flowing water
will modify nutrient and organic matter processing (Greenwald et al.,
2008; Zarnetske et al., 2008) but long-term negative impacts of increased
sediment load on biological productivity could outweigh any positive
effects from increased nutrient loading (Bowden et al., 2008).
Changes to river-ice flooding are also projected to occur as a result of
changes in (1) hydraulic gradients for near-coastal locations because of
sea level rise, (2) streamwise air-temperature gradients, and (3) the timing
and magnitude of spring snowmelt (Prowse et al., 2011). Synergistic/
antagonistic effects among these factors, however, require detailed site-
specific analyses for accurate projections of future conditions (Beltaos and
Prowse, 2009). Reduced (increased) ice-jam flooding will have positive
(negative) benefits for river-side northern communities/infrastructure
but could also alter delta-riparian (Lesack and Marsh, 2010) and coastal
marine (Emmerton et al., 2008) ecosystems. The quality of river water
entering the marine environment will also be affected by the reduction
or loss of stamukhi lakes that process river inputs (Dumas et al., 2006;
Galand et al., 2008).
Future changes to lake ice regimes will include delayed freeze-up,
advanced break-up, thinner ice and changes in cover composition
(especially white ice in areas of enhanced winter precipitation), increased
water temperature, and earlier and longer-lasting summer stratification
(Dibike et al., 2011), all of which will affect a range of aquatic processes,
including secondary productivity (Borgstrøm and Museth, 2005; Prowse
et al., 2007; Prowse and Brown, 2010b). Patterns of species richness and
diversity are also projected to change with alterations to ice duration—
i
ncreased open-water periods favoring the development of new trophic
levels, colonization of new aquatic species assemblages (Vincent et al.,
2009), greater atmosphere-water gas exchange, and a decrease in
winter kill of resident fish with cascading effects on lower trophic levels
(Balayla et al., 2010). The loss of ice, however, can also decrease key
habitat availability and quality (Vincent et al., 2008). Geochemical
responses of Arctic lakes will also be altered. As observed for thermokarst
lakes, the loss of ice cover and associated warming can greatly increase
methane production (Metje and Frenzel, 2007; Laurion et al., 2010).
Because temperature sensitivity has a stronger control over methane
production than oxidation (Duc et al., 2010), elevated water temperatures
will enhance methanogenesis, causing increased methane release from
sediments. The net balance of these two processes operating under a
broad range of future changing environmental factors, however, remains
to be quantified (Walter et al., 2007a,b, 2008; Laurion et al., 2010).
As well as methane, increased water temperatures are projected to lead
to reduced organic carbon (OC) burial. Projections, based on a range of
six climate warming scenarios (IPCC, 2007), indicate that there will be
a 4 to 27% decrease (0.9 to 6.4 TgC yr
–1
) in OC burial across lakes of
the northern boreal zone by the end of the 21st century as compared
to rates for the approximately last half-century (Gudasz et al., 2010).
Although these estimates assume that future OC delivery will be similar
to present-day conditions, even with enhanced supply from thawing
permafrost, higher water temperatures will increase OC mineralization
and thereby lower burial efficiency. The amount of burial also depends
on lake depth and mixing regimes. For non-thermally stratified shallow
lakes, there will be a greater opportunity for water-sediment mixing,
and hence greater carbon recycling back into the water column. By
contrast, for lakes that become increasingly thermally stratified, carbon
sinking below the thermocline will tend not to return to the surface until
an increasing later fall turnover, thereby decreasing the probability of
sediment-stored carbon being returned to the water column (Flanagan
et al., 2006).
Changes in ice cover, thermal regimes, and stratification patterns will
also affect the fate of contaminants in northern lakes. Higher water
temperatures can enhance the methylation of mercury and modify food
web and energy pathways, such as through enhanced algal scavenging
(a major food web entry pathway for mercury), resulting in increased
mercury bioavailability to higher trophic levels (Outridge et al., 2007;
Carrie et al., 2010).
28.3.1.2. Antarctica
This assessment reinforces conclusions of AR4. Increased temperatures
will impact aquatic ecosystems in Antarctica (high confidence), but the
exact nature of these impacts will vary regionally. The most vulnerable
freshwater systems are in the northern Antarctic Peninsula and maritime
Antarctic islands, where a small increase in temperature can have
widespread ecosystem impacts because the average temperature is
within a few degrees of the melting point (high confidence; Quesada and
Velázquez, 2012). Potential impacts are expected to range from immediate
catastrophic impacts such as loss of bounding ice masses causing
drainage of freshwater and epishelf lakes (Smith et al., 2006; Hodgson,
2011), to more gradual impacts on changes in the amount and duration

1587
Polar Regions Chapter 28
28
o
f catchment ice and snow cover; accelerated glacier melting; declining
volumes of precipitation falling as snow; permafrost; and active layer and
hydrological changes, such as water retention times (medium confidence;
e.g., Vieira et al., 2010; Quesada and Velázquez, 2012; Bockheim et al.,
2013).
Changes in the thickness and duration of seasonal ice cover, longer melt
seasons, and larger volumes of water flowing into the lakes are expected
in the future (medium confidence; Lyons et al., 2006) but the ecological
effects will vary between lakes, depending on their depth to surface
area ratio, with insufficient evidence to fully assess future changes in
these systems. Longer ice-free seasons may cause physical conditions
to be more favorable for primary production (Hodgson and Smol, 2008)
but very high irradiances experienced during summer in some systems
can substantially inhibit algal blooms under ice-free conditions (Tanabe
et al., 2007), which would favor the growth of benthic cyanobacteria
species (Hodgson et al., 2005). In other lakes, increases in meltwater
supply may increase suspended solids and reduce light penetration and
may offset the increases in the underwater light regime predicted as a
result of extended ice-free periods (Quesada et al., 2006).
Under a warming climate an increase in microbial biomass is expected
because of the increased water supply from glacial melt and warmer
temperatures, and could result in further development of soils and elevated
nutrient and dissolved OC delivery to lakes (Velázquez et al., 2013). This
organic supply will promote growth and reproduction in the benthos
and plankton and imbalances in population dynamics (Quesada and
Velázquez, 2013). Nutrient enrichment of some freshwater habitats in
the vicinity of fur seal colonies will increase because of expanding fur
seal populations (high confidence; Quayle et al., 2013).
Away from glacial forelands, increasing aridity will occur in the long
term in some areas of the continent (Hodgson et al., 2006b) and on
sub-Antarctic islands (medium confidence; Smith, Jr. et al., 2012). Closed
basin lakes can dry up completely causing local extinctions or retreat
into cryptic or resistant life-cycle stages, as experienced in Arctic lakes
(Smol and Douglas, 2007b). Other effects include desiccation of moss
banks due to increased evaporation and sublimation rates (medium
confidence; Wasley et al., 2006). Studies have also shown that warming
of once cold freshwater habitats in Antarctica will allow the sub- and
maritime Antarctic species to re-invade and establish self-maintaining
populations on the Antarctic continent, particularly where human
vectors are involved (medium confidence; Barnes et al., 2006; Hodgson
et al., 2006b). For other organisms with lower dispersal capabilities
there is increasing evidence of endemism, particularly in microbial
groups (Vyverman et al., 2010), with a possibility that surface Antarctic
lakes contain endemic species that are relics of Gondwana (cf. Convey
and Stevens, 2007) and that would become extinct should they be lost
from these lakes as a result of climate change.
28.3.2. Oceanography and Marine Ecosystems
28.3.2.1. Ocean Acidification in the Arctic and Antarctic
The effects of ocean acidification on polar marine food webs can have
considerable implications (medium confidence). For example, if some
r
egions in the Arctic become understaturated with respect to aragonite
(the primary structural component of the shells of some marine calcifiers
such as molluscs and urchins), the growth and survial of these organisms
will be impacted (WGI AR5 Figure 6.28; Chierici and Fransson, 2009; Fabry
et al., 2009; Yamamoto-Kawai et al., 2009). In laboratory experiments,
Arctic pteropods (Limacina helicina, a small planktonic mollusc) held under
conditions consistent with projected ocean warming and acidification
in the Arctic Ocean in early spring were able to extend their shells in
corrosive waters but dissolution marks were observed (Comeau et al.,
2010, 2012). Additional studies are needed to scale up regional impacts
to assess the population level impact of ocean acidification on Limacina
helicina and other vulnerable species (Orr et al., 2009). At the current time
there are insufficient data to fully assess the ecosystem consequences
of acidification on pteropods because it is unclear whether other
species, with a similar nutritive value, will replace pteropods.
In the Southern Ocean, foraminifera have thinner shells than in the
Holocene and there is evidence for shell thickness to be related to
atmospheric CO
2
, supporting the hypothesis that ocean acidification will
affect this abundant protozoan in this region (Moy et al., 2009). Similarly,
shells are thinner from sediment traps in aragonite undersaturated water
(below the aragonite saturation horizon (ASH)) compared to those
captured above the ASH in sub-Antarctic waters, but there is no time
series of data related to change in the ASH (Roberts et al., 2011). Shell
dissolution has been observed in surface waters in the Atlantic sector
as a result of both upwelling and atmospheric changes in CO
2
(medium
confidence; Bednarsek et al., 2012). Other impacts of acidification on
Southern Ocean organisms are currently uncertain, but short-term
negative impacts need to be considered together with an organism’s
capacity to adapt in the longer term (Watson et al., 2012).
Only a few studies have been conducted on commercially exploited polar
species on ocean acidification. Antarctic krill embryonic development
(Kawaguchi et al., 2011) and post-larval krill metabolic physiology (Saba
et al., 2012) may be impeded by elevated CO
2
concentrations, which
may negatively impact the reproductive success of krill more generally
under emission scenarios used in Coupled Model Intercomparison Project
Phase 5 (CMIP5) (medium confidence; Kawaguchi et al., 2013). Long
et al. (2013) examined the effects of acidification on red king crab
(Paralithodes camtschaticus) and found animals exposed to reduced pH
exhibited increased hatch duration, decreased egg yolk, increased larval
size, and decreased larval survival. In contrast, Hurst et al. (2012)
conducted laboratory experiments at levels of elevated CO
2
predicted to
be present in the Gulf of Alaska and Bering Sea in the next century and
found that juvenile walleye pollock (Gadus chalcogrammus) exhibited a
general resiliency of growth energetics to the direct effects of CO
2
changes.
28.3.2.2. Arctic
28.3.2.2.1. Marine plankton, fish, and other invertebrates
Phenological response
Projected changes in the timing, spatial distribution, and intensity of
spring blooms may result in mismatches with the timing of the emergence
of Arctic grazers (Søreide et al., 2010). Based on past experience,

1588
Chapter 28 Polar Regions
28
s
ome species will adapt to local conditions by shifting key life cycle
events (hatch date, maturity schedule, and reproductive timing) or diet to
accommodate differences in the regional timing and availability of prey
and environmental conditions (Ormseth and Norcross, 2007; Sundby
and Nakken, 2008; Vikebø et al., 2010; Darnis et al., 2012). For example,
loss of sea ice cover in spring is expected to change fish behavior in
ice-bound areas (Mundy and Evenson, 2011). It is uncertain whether
endemic animals will be able to alter key phenologies fast enough to
keep pace with the projected rates of change in the Arctic Ocean.
Projected spatial shifts
Simulation studies revealed that a 2-week longer growing season and
a 2°C increase in temperature would not be sufficient to allow expatriate
species (Calanus finmarchicus or C. marshallae) to invade the Arctic Ocean
(Ji et al., 2012). Ellingsen et al. (2008) projected future zooplankton
distribution and abundance in the Barents Sea for the period 1995–2059
using a regional climate model that was forced with climate model
output based on the Special Report on Emission Scenarios (SRES) B2
scenario. They projected that by 2059, Atlantic origin zooplankton will
increase and Arctic origin zooplankton will decrease in the Barents Sea.
The literature is mixed with respect to the potential for future movement
of fish and shellfish into the Arctic Ocean. Modeling studies project that
marine fish stocks potentially will shift their distributions into the Arctic
Ocean, resulting in an increase in biodiversity in the region (Cheung et
al., 2009, 2011; see also Box CC-MB). However, other studies show the
persistence of cold seawater temperatures on the shelf regions of the
Arctic Ocean and northern Bering Sea will restrict or retard movement
of several sub-Arctic fish and shellfish species into the Arctic Ocean
(Sigler et al., 2011; Stabeno et al., 2012b; Hunt, Jr. et al., 2013). In waters
off the coasts of Europe there is a potential for increased fish production
because of the combined effects of intrusion of Atlantic water over the
relatively broader shelf regions and advective corridors for larval drift
and range expansion of spawners. Huse and Ellingsen (2008) forced a
spatially explicit coupled biophysical model for the Barents Sea with
future climate scenarios to project the implications of climate change
on the spawning distribution of capelin (Mallotus villosus). Projections
show that the spawning distribution of capelin will shift to the east and
new spawning grounds will be colonized. A key factor governing this
expansion will be the availability of pelagic prey. In the southeast Bering
Sea, there is evidence that planktivorous species such as walleye pollock
will shift their distribution in response to shifts in ocean temperature
(Kotwicki and Lauth, 2013). In summary, the spatial distribution of some
fish and shellfish in the Barents and southeast Bering Seas will shift in
response to climate change (high confidence).
Projected impacts on production
In the deep basins of the Arctic Ocean the number of ice-free days in
summer are expected to result in longer productive seasons (high
confidence; Slagstad et al., 2011). Ellingsen et al. (2008) projected that
annual primary production would increase by 2059 in the Barents Sea.
Tremblay et al. (2012) hypothesized that longer ice-free periods in summer
in the Arctic Ocean could provide for more opportunities for episodic
n
utrient pulses that would enhance secondary production through the
growing season. However, in the Arctic Ocean, these changes in primary
production may be offset later in the year by increased zooplankton
grazing (Olli et al., 2007) or nutrient depletion due to stronger stratification
and shifts in the mixed layer depth (Wassmann, 2011; Tremblay et al.,
2012). Therefore, there is medium confidence that annual phytoplankton
production will increase in the central Arctic Ocean.
In the few cases where future abundance of fish has been projected
using climate change scenarios, species exhibited different trends related
to their vulnerability. Forward extrapolation of observed responses
suggests that increased summer sea surface temperatures in the Bering
and Barents Seas will cause a decrease in the abundance of energy-rich
copepods and euphausiids (Coyle et al., 2011; Slagstad et al., 2011).
This change in prey quality is expected to lower survival of walleye
pollock in the eastern Bering Sea by 2050 (Mueter et al., 2011). Climate-
enhanced stock projection models showed time trends in cross-shelf
transport of juvenile northern rock sole (Lepidopsetta polyxystra) to
nursery areas will not be substantially altered by climate change
(Wilderbuer et al., 2012).
28.3.2.2.2. Marine mammals, polar bears, and seabirds
The effects of the projected reduction in sea ice extent in this century
(Wang and Overland, 2009) on Arctic marine mammals and seabirds will
vary spatially and temporally (Laidre et al., 2008). Many ice-associated
marine mammals and seabirds will be affected by ice loss, with altered
species distributions, migration patterns, behavior, interspecific interactions,
demography, population changes, and vulnerability to extinction but
there is limited evidence of changes for most species (high confidence).
The polar bear population of the southern Beaufort Sea is projected to
decline by 99% by 2100, with a probability estimated at 0.80 to 0.94
under A1B (Hunter et al., 2010). The northern Beaufort Sea population
is stable although decline is predicted with warming (Stirling et al.,
2011). Projected extirpation of approximately two-thirds of the world’s
polar bears was predicted for mid-century under A1B (Amstrup et al.,
2008). Aspects of this study were criticized (Armstrong et al., 2008) but
refuted (Amstrup et al., 2009). The two-thirds decline is consistent with
other studies and has robust evidence with medium agreement. Projected
extinction of polar bears is unlikely. There is very high confidence of
subpopulation extirpation.
It is likely that the high Arctic seabird species partly or completely
dependent on the sympagic ecosystem or the cold Arctic waters close
to the ice edge will be negatively impacted if the projected changes in
these physical parameters occur (medium confidence). A general increase
in sea surface temperatures, retreat of the ice cover, and earlier break
up of fast ice may improve the environmental conditions and food
abundance for seabird species that have their range in the southern
part of the Arctic or south of the Arctic (medium confidence). A poleward
expansion of the range of these species is expected during a continued
warming (medium confidence).
Several factors other than climate influence seabird population dynamics
(Regular et al., 2010), and projections of changes with a continued Arctic

1589
Polar Regions Chapter 28
28
w
arming are therefore highly uncertain. Pattern of change will be non-
uniform and highly complex (ACIA, 2005). At present, the resolution of
Atmosphere-Ocean General Circulation Models are not detailed enough
to project spatial changes in mesoscale oceanographic features such
as frontal zones and eddies of importance to Arctic seabirds.
2
8.3.2.3. Antarctica and the Southern Ocean
Continued rising temperatures in the Southern Ocean will result in
increased metabolic costs in many ectothermic pelagic species, southward
movement of temperate species, and contraction of the range of polar
species (medium confidence). Southward movement of ocean fronts and
associated biota that are prey of sub-Antarctic island-based predators
will result in energetic inefficiencies for some of those predators (low
confidence; Péron et al., 2012; Weimerskirch et al., 2012).
For Antarctic krill, insufficient evidence is available to predict what will
happen to circumpolar productivity because of regional variability of the
effects of climate change on the different factors (positive and negative)
that affect krill, directly and indirectly. For example, increased metabolic
and growth rates from warming may be countered by a reduced food
supply and the effects of ocean acidification (Sections 28.2.2.2, 28.3.2.1).
Also, areas that are already warm may result in slower growth with
further warming, such as could happen in the northern Scotia Arc
(Wiedenmann et al., 2008; Hill et al., 2013). Models of recruitment and
population dynamics indicate that the biomass of krill will decline if
surface warming continues, but preliminary projections incorporating a
range of factors are uncertain (low confidence; Murphy et al., 2007,
2012b). Physiological and behavioral responses might also ameliorate
impacts. For example, krill are now known to exploit the full depth of
the ocean, which could provide escapes from further warming (Schmidt
et al., 2011) as well as refuge from air-breathing predators.
The strong dependence of species in more southern regions (e.g., southern
west Antarctic Peninsula and Ross Sea region) on sea ice means that
changes in sea ice distribution will cause spatial shifts in the structure
of ice-obligate food webs (low confidence; Murphy et al., 2012b).
Projections show that loss of summer sea ice from the west Antarctic
Peninsula is expected to result in ice-dependent seals declining and being
replaced by other seal species that are not dependent on sea ice (low
confidence; Siniff et al., 2008; Costa et al., 2010). There is insufficient
evidence to determine whether there will be a mismatch in phenologies
of different species as a result of changes in the winter sea ice season
(timing and winter extent), such as might occur if the timing of sea ice
melt was not at a time of optimal growing conditions for phytoplankton
(Trathan and Agnew, 2010).
Reductions in krill abundance in the marine food webs around the
South Atlantic islands may result in a shift in their structure toward a
more fish-centered ecosystem as observed in the Indian Sector (low
confidence; Trathan, et al., 2007, 2012; Shreeve et al., 2009; Waluda et
al., 2010; Murphy et al., 2012a,b). Also, salps have been postulated
to be competitors with krill for phytoplankton around the Antarctic
Peninsula when oceanic conditions displace shelf and near-shelf waters
during times of low sea ice (Ducklow et al., 2012). In the absence of
krill, longer food chains have lower trophic efficiency (Muprhy et al.,
2
013), and the long-term implications of this for higher trophic levels
are unknown.
Coastal environments will be impacted by the dynamics of fast ice, ice
shelves, and glacier tongues. These factors will positively affect local
primary production and food web dynamics (Peck et al., 2009) but
negatively affect benthic communities (low confidence; Barnes and
Souster, 2011). Projections of the response of emperor penguins and
Southern Ocean seabirds based on AR4 model outputs for sea ice and
temperature in east Antarctica indicate that general declines in these
populations are to be expected if sea ice habitats decline in the future
(low confidence; Barbraud et al., 2011; Jenouvrier et al., 2012). However,
these responses are also expected to be regionally specific because of
the regional differences in expectations of change in the ice habitats
(high confidence). Additional studies at other sites are needed to improve
confidence levels of predictions.
28.3.3. Terrestrial Environment and Related Ecosystems
28.3.3.1. Arctic
The boreal forest is generally projected by models to move northward
under a warming climate, which will displace between 11 and 50% of
the tundra within 100 years (Callaghan et al., 2005; Wolf et al., 2008;
Tchebakova et al., 2009; Wramneby et al., 2010) in a pattern similar to
that which occurred during the early Holocene climatic warming (high
confidence). Pearson et al. (2013) projected that at least half of vegetated
Arctic areas will shift to a different physiognomic class, and woody cover
will increase by as much as 52%, in line with what has been occurring
in northwest Eurasia (Macias-Fauria et al., 2012).
Dynamic vegetation models applied to Europe and the Barents Region
project a general increase in net annual primary production by climate
warming and CO
2
fertilization (Wolf et al., 2008; Wramneby et al., 2010;
Anisimov et al., 2011). Boreal needle-leaved evergreen coniferous forest
replaces tundra and expands into the mountain areas of Fennoscandia,
but this advance may be delayed or prevented in regions already
occupied by clonal deciduous shrubs whose in situ growth has increased
significantly in recent decades (Macias-Fauria et al., 2012).
In contrast to these expected results, shrubs, currently expanding in
area in many Arctic locations, were modeled to decrease in extent
over the next 100 years after an initial increase (Wolf et al., 2008). Also,
counterintuitively, tundra areas increased in the projections. This was a
result of changes at the highest latitudes that opened land for colonization
at a rate exceeding displacement of tundra by shrubs in the south.
Several studies have calculated the magnitude of the effects of vegetation
change in the Arctic on negative feedbacks of CO
2
sequestration and
increased evapotranspiration and the positive feedback of decreased
albedo (Swann et al., 2010; Wramneby et al., 2010; Wolf et al., 2010;
Pearson et al., 2013). It is likely that vegetation changes will result in
an overall positive feedback on the climate.
Recent changes and results of climate change simulation experiments
in the field have shown that there are considerable uncertainties in the

1590
Chapter 28 Polar Regions
28
p
rojected rates of change (e.g., Van Bogaert et al., 2010). Furthermore,
the models do not yet include vertebrate and invertebrate herbivory,
extreme events such as tundra fire, and extreme winter warming
damage or changes in land use that either reduce the rate of vegetation
change or open up niches for rapid change. Projections suggest increases
in the ranges of the autumn and winter months that have outbreaks in
populations resulting in the defoliation of birch forest (Jepsen et al.,
2008, 2011) and a general increase in the “background” (non-outbreak)
invertebrate herbivores (Wolf et al., 2008).
Animal terrestrial biodiversity is generally projected to increase in the
Arctic during warming by immigration of new species from the south,
vegetation changes, and indirectly by introduction of invasive species
caused by increased human activities and increased survival of such
species (high confidence; Post et al., 2009; Gilg et al., 2012; CAFF, 2013).
Many native Arctic species will likely be increasingly threatened during
this century.
28.3.3.2. Antarctica
Projected effects of climate change on Antarctic terrestrial species are
limited to knowledge of their ecophysiological tolerances to changes in
air temperature, wind speed, precipitation (rain and snowfall), permafrost
thaw, and exposure of new habitat through glacial/ice retreat. The climate
is expected to become more tolerable to a number of species, leading
to increases in biomass and extent of existing ecological communities.
The frequency with which new potential colonizing plant and animal
species arrive in Antarctica (particularly the Antarctic Peninsula region)
from lower latitudes, and the subsequent probability of their successful
establishment, will increase with regional climate warming and associated
environmental changes (high confidence; Chown et al., 2012). Human-
assisted transfers of biota may be more important by two orders of
magnitude than natural introductions (Frenot et al., 2005) as the transfer
is faster and avoids extreme environments such as altitude or oceans
(Barnes et al., 2006). The potential for anthropogenic introduction of
non-indigenous species to Antarctic terrestrial areas, which could have
devastating consequences to the local biodiversity, will increase (high
confidence; Convey et al., 2009; Hughes and Convey, 2010; Convey,
2011; Braun et al., 2012). At present, established non-indigenous species
in the sub- and maritime Antarctic are very restricted in their distributions
(Frenot et al., 2005). Climate change could result in a greater rate of
spread of invasive species through colonization of areas exposed by
glacial retreat, as has occurred at South Georgia (Cook et al., 2010) and
in the maritime Antarctic (Olech and Chwedorzewska, 2011). Biosecurity
measures may be needed to help control dispersal of established non-
indigenous species to new locations, particularly given the expected
increase in human activities in terrestrial areas (Hughes and Convey, 2010;
Convey et al., 2011). An important gap in understanding is the degree
to which climate change may facilitate some established but localized
alien species to become invasive and widespread (Frenot et al., 2005;
Convey 2010; Hughes and Convey, 2010; Cowan et al., 2011), which
has been shown for the sub-Antarctic (Chown et al., 2012).
Overall, the likely impacts of existing and new non-indigenous species
on the native terrestrial ecosystems of Antarctica and the sub-Antarctic
i
slands, along with the continued increased presence of Antarctic fur
seals, are likely to have far greater importance over the time scale
under consideration than are those attributable to climate change itself
(Convey and Lebouvier, 2009; Turner et al., 2009; Convey, 2010).
28.3.4. Economic Sectors
Projections of economic costs of climate change impacts for different
economic sectors in the Arctic are limited, but current assessments
suggest that there will be both benefits and costs (AMAP, 2011a;
Forbes, 2011). Non-Arctic actors are likely to receive most of the benefits
from increased shipping and commercial development of renewable
and non-renewable resources, while Indigenous peoples and local
Arctic communities will have a harder time maintaining their way of
life (Hovelsrud et al., 2011).
Contributing to the complexity of measuring the future economic effects
of climate change is the uncertainty in future predictions and the
rapid speed of change, which are linked with the uncertainty of the
technological and ecological effects of such change (NorAcia, 2010).
Communities within the same eco-zone may experience different effects
from identical climate-related events because of marked local variations
in site, situation, culture, and economy (Clark et al., 2008).
Economic cost estimates have been made for the case of the Alaskan
economy, for example, which suggest that a heavy reliance on climate-
sensitive businesses such as tourism, forestry, and fisheries renders the
economy vulnerable to climate change, and that Alaska Native peoples,
reliant on the biodiversity of the Alaskan ecosystem, are being affected
disproportionately (Epstein and Ferber, 2011). Some Alaskan villages
such as Shishmaref, Kivalina, and Newtok have already lost critical
infrastructure and services and are becoming unlivable because of
permafrost thaw, storm damage, and coastal erosion but the high costs
and limitations of government mechanisms are significant barriers to
the actual relocation of these communities (Bronen, 2011; Brubaker et
al., 2011c; Cochran et al., 2013; Maldonado et al., 2013).
28.3.4.1. Fisheries
Climate change will impact the spatial distribution and catch of some
open ocean fisheries in the Barents and Bering Seas (high confidence);
however, the future of commercial fisheries in the Arctic Ocean is uncertain.
There is strong evidence and considerable data showing links between
climate-driven shifts in ocean conditions and shifts in the spatial distribution
and abundance of commercial species in the Bering and Barents Seas
(Section 28.3.2.2.1). In limited cases, coupled biophysical models or
climate-enhanced stock projection models have been used to predict
future commercial yield or shifts in fishing locations. However, these
predictions are uncertain (Huse and Ellingsen, 2008; Ianelli et al., 2011;
Wilderbuer et al., 2012). Cheung et al. (2011) used projections from an
Earth System Model to estimate shifts in bio-climatic windows that
included climate change effects on biogeochemistry (oxygen and acidity)
and primary production to project future catch potential of 120 demersal
fish and invertebrates. Results from their model suggested that the catch
potential will increase in the Barents and Greenland Seas and regions
1591
Polar Regions Chapter 28
28
a
t greater than 70° north latitude (Cheung et al., 2011). In contrast,
vulnerability analysis suggests that only a few species are expected to
be abundant enough to support viable fisheries in the Arctic Ocean
(Hollowed et al., 2013). Potential fisheries for snow crab (Chionoecetes
opilio) on shelf areas of the Arctic Ocean may be limited by the associated
impacts of ocean acidification. If fisheries develop in the Arctic Ocean,
adoption of sustainable strategies for management will be a high
priority (Molenaar, 2009). The moratorium on fishing in the US portion
of the Chukchi and Beaufort Seas would prevent fishing until sufficient
data become available to manage the stock sustainably (Wilson and
Ormseth, 2009).
Predicting how harvesters will respond to changing economic, institutional,
and environmental conditions under climate change is difficult. Current
techniques track fishers’ choices based on revenues and costs associated
with targeting a species in a given time and area with a particular gear
given projected changes in the abundance and spatial distribution of
target species (Haynie and Pfeiffer, 2012). However, estimates of future
revenues and costs will depend, in part, on future demand for fish, global
fish markets, and trends in aquaculture practices (Rice and Garcia, 2011;
Merino et al., 2012).
28.3.4.2. Forestry and Farming
Climate change is likely to have positive impacts for agriculture,
including extended growing season (medium to high confidence;
Falloon and Betts, 2009; Grønlund, 2009; Tholstrup and Rasmussen,
2009), although variations across regions are expected (Hovelsrud et
al., 2011), and the importance of impacts to the Arctic economy will
likely remain minor (Eskeland and Flottorp, 2006). Potential positive
effects of climatic warming for forestry include decreased risk of snow
damage. Kilpeläinen et al. (2010) estimate a 50% decrease in snow
damage in Finland toward the end of the century. A warmer climate is
likely to impact access conditions and plant diseases for forestry and
farming. Grønlund (2009) found in the case of northern Norway—
where about half of the arable land area is covered by forest and 40%
by marshland—that the potential harnessing of arable land for farming
will be at the cost of forestry production, or dried-up marshlands, which
may contribute to more greenhouse emissions. Larger field areas may
contribute to land erosion through rainfall and predicted unstable
winters, and may increase conditions for plant diseases and fungal
infections (Grønlund, 2009). If the winter season continues to shorten
due to climate change (Xu et al., 2013), accessibility to logging sites
will be negatively affected. Accessibility is higher when frozen ground
makes transportation possible in sensitive locations or areas that lack
road. If weather changes occur when logging has taken place, sanding
of roads may be necessary which carries significant economic costs.
Impact on carrying capacity of ground or road accessibility will thus
affect forestry economically. Challenges may include limited storage
space for wood (Keskitalo, 2008).
28.3.4.3. Infrastructure, Transportation, and Terrestrial Resources
Rising temperatures and changing precipitation patterns have the
potential to affect all infrastructure types and related services, as much
o
f the infrastructure in the North is dependent on the cryosphere to, for
example, provide stable surfaces for buildings and pipelines, contain
waste, stabilize shorelines, and provide access to remote communities
in the winter (high confidence; Huntington et al., 2007; Furgal and
Prowse, 2008; Sundby and Nakken, 2008; Sherman et al., 2009; West
and Hovelsrud, 2010; Forbes, 2011). In the long-term, marine and
freshwater transportation will need to shift reliance from ice routes to
open-water or land-based transportation systems. Relocation remains
one community-based adaptation to deal with projections of persistent
flooding and bank erosion (Furgal, 2008; NRTEE, 2009). Changing sea
ice (multi-year) conditions are expected to have a regulating impact on
marine shipping and coastal infrastructure (i.e., via introduced hazards;
Eicken et al., 2009).
By adapting transportation models to integrate monthly climate
model (Community Climate System Model 3 (CCSM3)) predictions of
air temperature—combined with data sets on land cover, topography,
hydrography, built infrastructure, and locations of human settlements—
estimates have been made of changes to inland accessibility for
landscapes northward of 40ºN by the mid-21st century (Stephenson et
al., 2011). Milder air temperatures and/or increased snowfall reduce
the possibilities for constructing inland winter-road networks, including
ice roads, with the major seasonal reductions in road potential (based
on a 2000-kg vehicle) being in the winter shoulder-season months of
November and April. The average decline (compared to a baseline of
2000–2014) for eight circumpolar countries was projected to be
–14%, varying from –11 to –82%. In absolute terms, Canada and Russia
(both at –13%) account for the majority of declining winter-road
potential with approximately 1 × 10
6
km
2
being lost (see Table 28-1).
The winter road season has decreased since the 1970s on the Alaskan
North Slope, from as much as 200 to 100 days in some areas (Hinzman,
et al., 2005).
Climate change is expected to lead to a nearly ice-free Arctic Ocean in
late summer and increased navigability of Arctic marine waters within
this century. New possibilities for shipping routes and extended use
of existing routes may result from increased melting of sea ice (high
confidence; Corbett et al., 2010; Khon et al., 2010; Paxian et al., 2010;
Peters et al., 2011; Stephenson et al., 2011).
Change (%) in winter road-
accessible land area (km
2
)
(2000-kg GVWR vehicle)
Change (%) in maritime-
accessible ocean area (km
2
)
(type A vessel) — current EEZ
Canada – 13 19
Finland – 41 0
Greenland – 11 28
Iceland – 82 < 1
Norway – 51 2
Russia – 13 16
Sweden – 46 0
USA (Alaska) – 29 5
High seas n /a 406
Total – 14 23
Table 28-1 | Annually averaged changes in inland and maritime transportation
accessibility by mid-century (2045 – 2059) versus baseline (2000 – 2014).
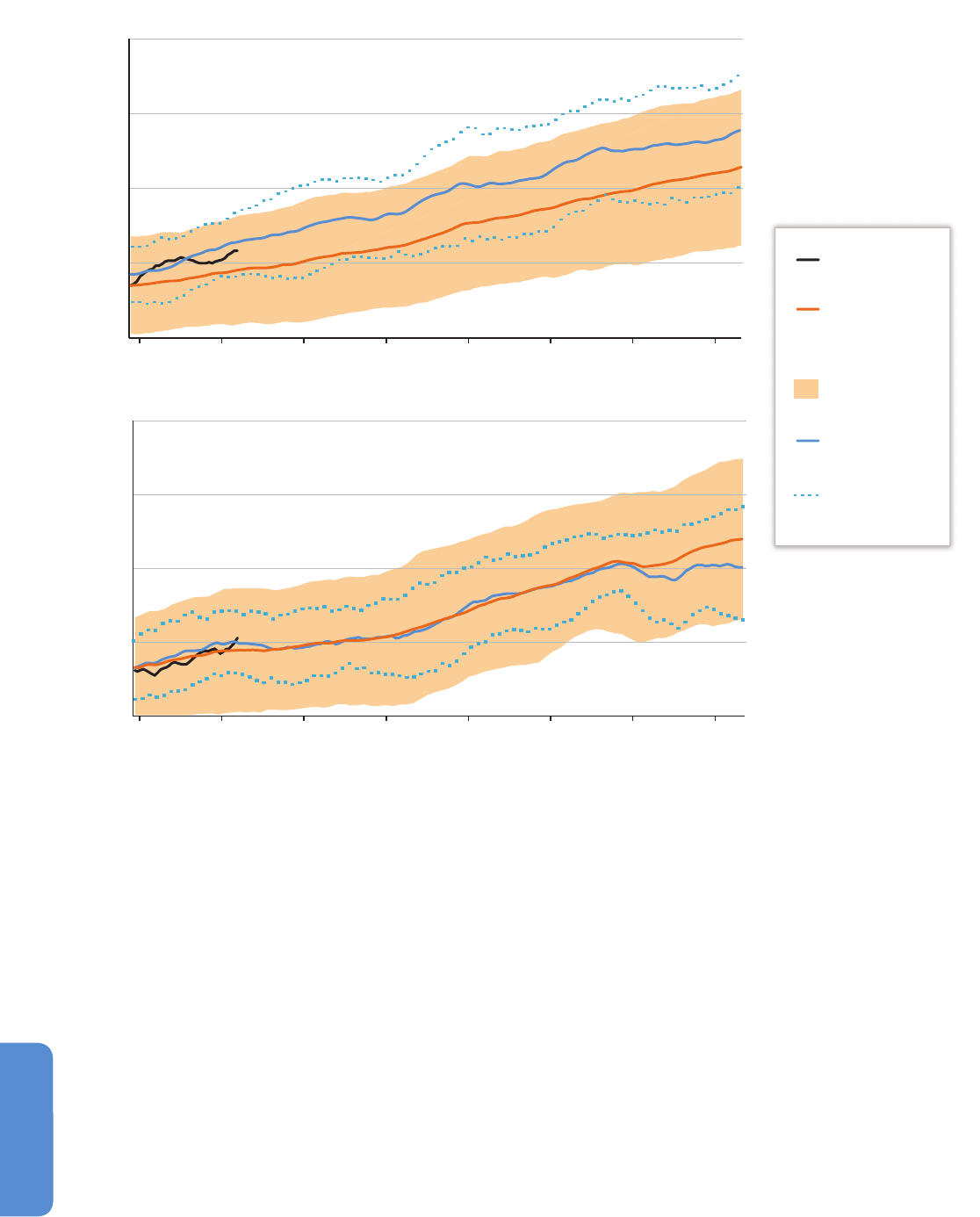
1592
Chapter 28 Polar Regions
28
Projections made by Stephenson et al. (2011) suggest that all five Arctic
littoral states will gain increased maritime access to their current exclusive
economic zones, especially Greenland (+28%, relative to baseline),
Canada (+19%), Russia (+16%), and the USA (+15%). In contrast,
Iceland, Norway, Sweden, and Finland display little or no increase in
maritime accessibility (Table 28-1; Stephenson et al., 2011).
General Circulation Models (GCMs) developed for the AR4 generally
have underestimated the duration of the ice-free period in the Arctic
Ocean and simulate slower changes than those observed in the past
decades (Stroeve et al., 2007). Mokhow and Khon (2008) used a subset
of climate models that better reproduce observed sea ice dynamics than
other GCMs to project the duration of the navigation season along the
NSR and through the NWP under the moderate SRES A1B emission
scenario. According to their results, by the end of the 21st century, the
NSR may be open for navigation 4.5 ± 1.3 months per year, while the
NWP may be open 2 to 4 months per year (see Figure 28-4). The models
did not predict any significant changes of the ice conditions in the NWP
until the early 2030s.
An increase in the length of the summer shipping season, with sea ice
duration expected to be 10 days shorter by 2020 and 20 to 30 days
shorter by 2080, is likely to be the most obvious impact of changing
climate on Arctic marine transportation (Prowse et al., 2009). Reduction
in sea ice and increased marine traffic could offer opportunities for
economic diversification in new service sectors supporting marine
shipping. Loss of sea ice may open up waterways and opportunities for
increased cruise traffic (e.g., Glomsrød and Aslaksen, 2009), and add to
an already rapid increase in cruise tourism (Howell et al., 2007; Stewart
et al., 2007, 2010). Climate change has increased the prevalence of
cruise tourism throughout Greenland, Norway, Alaska, and Canada
because of decreasing sea ice extent.
Projected declines in sea ice cover leading to development of integrated
land and marine transportation networks in northern Canada may
stimulate further mine exploration and development (Prowse et al., 2009).
These possibilities, however, also come with challenges including their
predicted contribution to the largest change in contaminant movement
into or within the Arctic, as well as their significant negative impacts
0
50
100
1
50
2
00
0
50
100
150
200
1985 2000 2015 2030 2045 2060 2075 2090
1985 2000 2015 2030 2045 2060 2075 2090
Navigation season (days)
(a) Northern Sea Route
Navigation season (days)
(b) Northwest Passage
Navigation season
length from
satellite data
Navigation season
length estimated
from all analyzed
models
Inter-model
standard
deviations
Navigation season
length estimated
from “best” models
Inter-model
standard
deviations
Figure 28-4 | Projected duration of the navigation period (days) over the Northwest Passage and Northern Sea Route (Khon et al., 2010).

1593
Polar Regions Chapter 28
28
o
n the traditional ways of life of northern residents (Furgal and Prowse,
2008). Added shipping and economic activity will increase the amount
of black carbon and reinforce warming trends in the region (Lack and
Corbett, 2012), leading to additional economic activity.
A longer shipping season and improved access to ports may lead to
increased petroleum activities, although possible increased wave activity
and coastal erosion may increase costs related to infrastructure and
technology. Peters et al. (2011) find by using a bottom-up shipping
model and a detailed global energy market model to construct emission
inventories of Arctic shipping and petroleum activities in 2030 and
2050—and based on estimated sea ice extent—that there will be rapid
growth in transit shipping; oil and gas production will be moving into
locations requiring more ship transport; and this will lead to rapid
growth in emissions from oil and gas transport by ship.
The Arctic contains vast resources of oil, which is hard to replace as
transportation fuel, and vast resources of gas, a more climate-benign
fuel than coal. Petroleum resources are unevenly distributed among
Arctic regions and states. Arctic resources will play a growing role in
the world economy, but increased accessibility is expected to create
challenges for extraction, transport, engineering, search-and-rescue
needs, and responses to accidents (Hovelsrud et al., 2011), and climatic
change presents the oil and gas industry with challenges in terms of
planning and predictions (Harsem et al., 2011). Increased emissions
due to rapid growth in Arctic Ocean transportation of oil and gas are
projected (Peters et al., 2011). Owing to high costs and difficult access
conditions, the impact on future oil and gas production in the Arctic
remains unclear (Peters et al., 2011; Lindholdt and Glomsrød, 2012).
28.4. Human Adaptation
There is general agreement that both Indigenous and non-Indigenous
people in the Arctic have a history of adapting to natural variability in
the climate and natural resource base, as well as recent socioeconomic,
cultural, and technological changes (high confidence; Forbes and Stammler,
2009; Wenzel, 2009; Ford and Pearce, 2010; West and Hovelsrud, 2010;
Bolton et al., 2011; Cochran et al., 2013). Climate change exacerbates
the existing stresses faced by Arctic communities (high confidence; Crate
and Nuttall, 2009; Rybråten and Hovelsrud, 2010), and is only one of many
important factors influencing adaptation (Berrang-Ford et al., 2011).
Climate adaptation needs to be seen in the context of these interconnected
and mutually reinforcing factors (Tyler et al., 2007; Hovelsrud and Smit,
2010). The challenges faced today by communities in the Arctic are
complex and interlinked and are testing their traditional adaptive capacity
(low to medium confidence).
Climatic and other large-scale changes have potentially large effects
on Arctic communities, in particular where simple economies leave a
narrower range of adaptive choices (Berkes et al., 2003; Anisimov et al.,
2007; Ford and Furgal, 2009; Andrachuk and Pearce, 2010; Ford et al.,
2010; Forbes, 2011). There is considerable evidence that changing
weather patterns, declining sea ice and river as well as lake ice, thawing
permafrost, and plant and animal species’ abundance and composition
have consequences for communities in the Arctic (see Sections 28.2.4,
28.2.5.2, and 28.3.4). Sea ice is particularly important for coastal
c
ommunities that rely upon it for transportation to and from hunting
areas (Krupnik et al., 2010). Changes in the duration and condition of sea
ice and the consequent changes to country food availability significantly
impact the well-being of communities (Furgal and Seguin, 2006; Ford
and Berrang-Ford, 2009; Ford et al., 2010), outdoor tourism (Dawson et
al., 2010), and hunting and fishing (high confidence; Wiig et al., 2008;
Brander, 2010).
Adaptation to climate change is taking place at the local and regional
levels where impacts are often felt most acutely and the resources most
readily available (Oskal, 2008; Hovelsrud and Smit, 2010). Current
experiences and projections of future conditions often lead to technological
adaptation responses such as flood and water management and snow
avalanche protection (Hovelsrud and Smit, 2010; West and Hovelsrud,
2010) rather than policy responses (Hedensted Lund et al., 2012;
Rudberg et al., 2012). Climate variability and extreme events are found
to be salient drivers of adaptation (Amundsen et al., 2010; Berrang-Ford
et al., 2011; Dannevig et al., 2012).
The lack of local scale climate projections, combined with uncertainties
in future economic, social, and technological developments, often act
as barriers to adaptation. These barriers, together with other societal
determinants such as ethics, cultures, and attitudes toward risk, may
cause inaction (Adger et al., 2009; West and Hovelsrud, 2010). Resolving
divergent values across and within different communities poses a
challenge for governance regimes. A determining factor in building
adaptive capacity is the flexibility of enabling institutions to develop
robust options (Forbes et al., 2009; Keskitalo et al., 2009; Hovelsrud and
Smit, 2010; Ford and Goldhar, 2012; Whyte, 2013). Refer to Table 28-2
for key climate-related risks and potential adaptation practices. In the
North American and Scandinavian context, adaptive co-management
responses have been developed through land claims settlements and/or
multi-scale institutional cooperation to foster social learning (Armitage
et al., 2008; Berkes, 2009).
Indigenous Peoples
Although Arctic indigenous peoples with traditional lifestyles are facing
unprecedented impacts to their ways of life from climate change and
resource development (oil and gas, mining, forestry, hydropower, tourism,
etc.), they are already implementing creative ways of adapting (high
confidence; Cruikshank, 2001; Forbes et al., 2006; Krupnik and Ray,
2007; Salick and Ross, 2009; Green and Raygorodetsky, 2010; Alexander
et al., 2011; Cullen-Unsworth et al., 2011).
While many of these adaptation activities tend to be short term or
reactive in nature (e.g., dealing with other issues such as disaster response
planning), some Indigenous communities are beginning to develop
more formal adaptation plans (Galloway-McLean, 2010; Brubaker et al.,
2011b,c; Nakashima et al., 2012). Comprehensive adaptation planning
must take into account underlying social issues of some Indigenous
populations when addressing the new challenges from climate and
development. Indigenous communities are especially vulnerable to climate
change because of their strong dependence on the environment for
food, culture, and way of life; their political and economic marginalization;
the social, health, and poverty disparities; and community locations
1594
Chapter 28 Polar Regions
28
along exposed ocean, lake, or river shorelines (Ford and Furgal, 2009;
Galloway-McLean, 2010; Larsen et al., 2010; Cochran et al., 2013).
The adaptive capacity of Arctic Indigenous peoples is largely due to an
extensive traditional knowledge and cultural repertoire, and flexible
social networks (medium confidence; Williams and Hardison, 2013; see
Section 12.3). The dynamic nature of traditional knowledge is valuable
for adapting to current conditions (Kitti et al., 2006; Tyler et al., 2007; Eira
et al., 2012). The sharing of knowledge ensures rapid responses to crises
(Ford et al., 2007). In addition, cultural values such as sharing, patience,
persistence, calmness, and respect for elders and the environment are
important. Some studies suggest that traditional knowledge may not
always be sufficient to meet the rapid changes in climate (see Chapter
12) and it may be perceived to be less reliable because the changing
conditions are beyond the current knowledge range (Ingram et al., 2002;
Ford et al., 2006; Hovelsrud et al., 2010; Valdivia et al., 2010).
Over the last half-century, the adaptive capacity in some Indigenous
communities has been challenged by the transition from semi-nomadic
hunting groups to permanent settlements, accompanied by impacts to
health and well-being from loss of connection to the land, traditional
foods, and culture (Ford et al., 2010; Galloway-McLean, 2010). Forced
or voluntary migration as an adaptation response can have deep
cultural impacts (Shearer, 2011, 2012; Maldonado et al., 2013). On the
other hand, the establishment of permanent communities, particularly
those associated with new industrial development, can also lead to
increasing employment opportunities and income diversification for
Indigenous peoples. The intergenerational transfers of knowledge
and skills through school curricula, land camps, and involvement in
community-based monitoring programs may strengthen adaptive
capacity (Forbes 2007; Ford et al., 2007; Hovelsrud and Smit, 2010;
Bolton et al., 2011).
Examples of Indigenous adaptation strategies have included changing
resource bases; shifting land use and/or settlement areas; combining
technologies with traditional knowledge; changing timing and location
of hunting, gathering, herding, and fishing areas; and improving
communications and education (Galloway-McLean, 2010). Protection
of grazing land will be the most important adaptive strategy for reindeer
herders under climate change (Forbes et al., 2009; Magga et al., 2011;
Kumpula et al., 2012; Degteva and Nellemann, 2013; Mathiesen et al.,
2013). Renewable resource harvesting remains a significant component
of Arctic livelihoods, and with climate change hunting and fishing has
become a riskier undertaking and many communities are already adapting
(Gearheard et al., 2011; Laidler et al., 2011). Adaptation includes taking
more supplies when hunting, constructing permanent shelters on land
as refuges from storms, improved communications infrastructure, greater
use of global positioning systems (GPS) for navigation, synthetic aperture
radar (SAR) to provide estimates of sea ice conditions (Laidler et al.,
2011), and the use of larger or faster vehicles (Ford et al., 2010). Avoiding
dangerous terrain can result in longer and time-consuming journeys
that can be inconvenient to those with wage-earning employment (Ford
et al., 2007).
Reindeer herders have developed a wide range of adaptation strategies
in response to changing pasture conditions. These include moving herds
to better pastures (Bartsch et al., 2010), providing supplemental feeding
(Helle and Jaakkola, 2008; Forbes and Kumpula, 2009), retaining a few
castrated reindeer males to break through heavy ice crust (Oskal, 2008;
Reinert et al., 2008), ensuring an optimal herd size (Tyler et al., 2007;
Ocean
acidification
C
OO
Climate-related drivers of impacts
Warming
trend
L
evel of risk & potential for adaptation
Potential for additional adaptation
to reduce risk
Risk level with
c
urrent adaptation
Risk level with
h
igh adaptation
Snow
cover
Table 28-2 |Key climate-related risks in the Arctic and Antarctic, and potential adaptation practices.
Very
low
Very
high
Medium
Key risk Adaptation issues & prospects
Climatic
drivers
Risk & potential for
adaptation
Timeframe
Near term
(
2030–2040)
P
resent
L
ong term
(
2080–2100)
2°C
4°C
Very
low
Very
high
Medium
N
ear term
(2030–2040)
Present
Long term
(2080–2100)
2°C
4°C
Very
low
Very
high
Medium
C
O
O
R
isks for freshwater and terrestrial ecosystems
(
high confidence) and marine ecosystems
(
medium confidence), due to changes in ice,
s
now cover, permafrost, and freshwater/ocean
c
onditions, affecting species´ habitat quality,
r
anges, phenology, and productivity, as well
a
s dependent economies
[
28.2-4]
•
Improved understanding through scientific and indigenous knowledge,
p
roducing more effective solutions and/or technological innovations
•
Enhanced monitoring, regulation, and warning systems that achieve safe and
s
ustainable use of ecosystem resources
• Hunting or fishing for different species, if possible, and diversifying income
sources
Risks for the health and well-being of Arctic
residents, resulting from injuries and illness
from the changing physical environment,
food insecurity, lack of reliable and safe
drinking water, and damage to
infrastructure, including infrastructure in
permafrost regions (high confidence)
[28.2-4]
• Co-production of more robust solutions that combine science and technology
with indigenous knowledge
• Enhanced observation, monitoring, and warning systems
• Improved communications, education, and training
•
Shifting resource bases, land use, and/or settlement areas
N
ear term
(2030–2040)
Present
Long term
(2080–2100)
2°C
4°C
U
nprecedented challenges for northern
c
ommunities due to complex inter-linkages
b
etween climate-related hazards and societal
f
actors, particularly if rate of change is faster
t
han social systems can adapt
(
high confidence)
[28.2-4]
•
Co-production of more robust solutions that combine science and
t
echnology with indigenous knowledge
•
Enhanced observation, monitoring, and warning systems
•
Improved communications, education, and training
• Adaptive co-management responses developed through the settlement of
land claims

1595
Polar Regions Chapter 28
28
F
orbes et al., 2009), and creating multicultural initiatives combining
traditional with scientific knowledge (Vuojala-Magga et al., 2011). Coastal
fishers have adapted to changing climate by targeting different species
and diversifying income sources (Hovelsrud et al., 2010).
In some Arctic countries Indigenous peoples have successfully negotiated
land claims rights and have become key players in addressing climate
change (Abele et al., 2009). In some instances, this has given rise to
tensions over land/water use between traditional livelihoods and new
opportunities, for example, tourism and natural resource development
(Forbes et al., 2006; Hovelsrud and Smit, 2010). Some territorial
governments in northern Canada have promoted adaptation by providing
hunter support programs (Ford et al., 2006, 2010).
Health of many Indigenous people is being affected by the interaction
of changes in the climate with ongoing changes in human, economic,
and biophysical systems (Donaldson et al., 2010). The distribution of
traditional foods between communities and the use of community
freezers in the Canadian Arctic has improved food security, an important
factor for health (Ford et al., 2010). Although wage employment may
e
nhance the possibilities for adaptive capacity, greater involvement in
full-time jobs can threaten social and cultural cohesion and mental well-
being by disrupting the traditional cycle of land-based practices (Berner
et al., 2005; Furgal, 2008).
28.5. Research and Data Gaps
There remains a poor knowledge of coupling among, and thresholds
within, biogeophysical and socioeconomic processes to fully assess the
effects ofa changing climate, and to separate them from those due to
other environmental stressors:
• Existing integrative models are either lacking or insufficiently
validated to project and to assess the cascading effects on, and
feedbacks from, the systems in the polar regions, in particular
socioeconomic systems.
• There is a need to enhance or establish a coordinated network of
long-term representative sites for monitoring and assessment of
climate change detection and attribution studies in the polar regions.
Regional differences and confounding variables will need to be
Frequently Asked Questions
FAQ 28.1 | What will be the net socioeconomic impacts of change in the polar regions?
Climate change will have costs and benefits for polar regions. Climate change, exacerbated by other large-scale
changes, can have potentially large effects on Arctic communities, where relatively simple economies leave a narrower
range of adaptive choices.
In the Arctic, positive impacts include new possibilities for economic diversification, marine shipping, agricultural
production, forestry, and tourism. The Northern Sea Route is predicted to have up to 125 days per year suitable for
navigation by 2050, while the heating energy demand in the populated Arctic areas is predicted to decline by 15%.
In addition, there could be greater accessibility to offshore mineral and energy resources although challenges related
to environmental impacts and traditional livelihoods are possible.
Changing sea ice condition and permafrost thawing may cause damage to bridges, pipelines, drilling platforms,
hydropower, and other infrastructure. This poses major economic costs and human risks, although these impacts are
closely linked to the design of the structure. Furthermore, warmer winter temperatures will shorten the accessibility
of ice roads that are critical for communications between settlements and economic development and have
implications for increased costs. Statistically, a long-term mean increase of 2°C to 3°C in autumn and spring air
temperature produces an approximately 10- to 15-day delay in freeze-up and advance in break-up, respectively.
Particular concerns are associated with projected increase in the frequency and severity of ice-jam floods on Siberian
rivers. They may have potentially catastrophic consequences for the villages and cities located in the river plain, as
exemplified by the 2001 Lena River flood, which demolished most of the buildings in the city of Lensk.
Changing sea ice conditions will impact Indigenous livelihoods, and changes in resources, including marine mammals,
could represent a significant economic loss for many local communities. Food security and health and well-being
are expected to be impacted negatively.
In the Antarctic, tourism is expected to increase, and risks exist of accidental pollution from maritime accidents,
along with an increasing likelihood of the introduction of alien species to terrestrial environments. Fishing for
Antarctic krill near the Antarctic continent is expected to become more common during winter months in areas
where there is less winter sea ice.

1596
Chapter 28 Polar Regions
28
considered in designing field and modeling studies. Standardized
methods and approaches of biophysical and socioeconomic analysis
along with coordinated sampling in more regions will be necessary.
There are more specific research gaps, including:
• Many mechanisms of how climate change and ocean acidification
may be affecting polar ecosystems have been proposed but few
studies of physiological tolerances of species, long-term field studies
of ecosystem effects, and ecosystem modeling studies are available
to be able to attribute with high confidence current and future
change in these ecosystems to climate change.
• More comprehensive studies including long-term monitoring on the
increasing impacts from climate changes on Arctic communities
(urban and rural) and their health, well-being, traditional livelihoods,
and life ways are needed. There is a need to assess more fully
vulnerabilities and to develop response capacities at the local and
regional levels.
References
Aaheim, A., H. Dannevig, T. Ericsson, B. van Oort, L. Innbjør, T. Rauken, H. Vennemo, H.
Johansen, M. Tofteng, C. Aall, K. Groven, and E. Heiberg, 2009. Konsekvenser av
Klimaendringer, Tilpasning og Sårbarhet i Norge – Rapport Klimatilpasningsutvalget.
CICERO Report 2009:04, Center for International Climate and Environmental
Research (CICERO), Oslo, Norway, 228 pp. (in Norwegian).
Abele, F., T.J. Courchene, F.L. Sidele, and F. St-Hilaire (eds.), 2009: Northern Exposure:
Peoples, Powers and Prospects in Canada’s North. Art of the State Series, Vol. 4,
The Institute for Research on Public Policy (IRPP) and McGill-Queen’s University
Press, Montréal, QC, Canada, 605 pp.
Abryutina, L.I., 2009: Indigenous peoples of the Russian North: social and climatic
changes. In: Climate Change and Arctic Sustainable Development: Scientific, Social,
Cultural, and Educational Challenges. United Nations Educational, Scientific,
and Cultural Organization (UNESCO), Paris, France, pp. 164-173.
Adam, J.C., A.F. Hamlet, and D.P. Lettenmaier, 2009: Implications of global climate
change for snowmelt hydrology in the twenty-first century. Hydrological
Processes, 23(7), 962-972.
Adger, W., S. Dessai, M. Goulden, M. Hulme, I. Lorenzoni, D. Nelson, L. Naess, J. Wolf,
and A. Wreford, 2009: Are there social limits to adaptation to climate change?
Climatic Change, 93(3), 335-354.
Albrecht, G., G.-M. Sartore, L. Connor, N. Higginbotham, S. Freeman, B. Kelly, H. Stain,
A. Tonna, and G. Pollard, 2007: Solastalgia: the distress caused by environmental
change. Australasian Psychiatry, 15(Suppl.), S95-S98.
Alexander, C., N. Bynum, E. Johnson, U. King, T. Mustonen, P. Neofotis, N. Oettlé, C.
Rosenzweig, C. Sakakibara, V. Shadrin, M. Vicarelli, Jon Waterhouse, and B.
Weeks, 2011: Linking indigenous and scientific knowledge of climate change.
BioScience, 61(6), 477-484.
AMAP, 2009: AMAP Assessment 2009: Human Health in the Arctic. Arctic Monitoring
and Assessment Programme (AMAP), Oslo, Norway, 254 pp.
AMAP, 2010: AMAP Assessment 2009: Radioactivity in the Arctic. Arctic Monitoring
and Assessment Programme (AMAP), Oslo, Norway, 92 pp.
AMAP, 2011a: Snow, Water, Ice and Permafrost in the Arctic (SWIPA): Climate Change
and the Cryosphere. Arctic Monitoring and Assessment Programme (AMAP),
Oslo, Norway, 538 pp.
AMAP, 2011b: Arctic Pollution 2011. Arctic Monitoring and Assessment Programme
(AMAP), Oslo, Norway, 38 pp.
Amstrup, S.C., I. Stirling, T.S. Smith, C. Perham, and G.W. Thiemann, 2006: Recent
observations of intraspecific predation and cannibalism among polar bears in
the southern Beaufort Sea. Polar Biology, 29(11), 997-1002.
Amstrup, S.C., B.G. Marcot, and D.C. Douglas, 2008: A Bayesian network approach
to forecasting the 21
st
century worldwide status of polar bears. In: Arctic Sea
Ice Decline: Observations, Projections, Mechanisms, and Implications
[DeWeaver, E.T., C.M. Bitz, and L.B. Tremblay (eds.)]. American Geophysical
Union (AGU), Washington, DC, USA, 213 pp.
Frequently Asked Questions
FAQ 28.2 | Why are changes in sea ice so important to the polar regions?
Sea ice is a dominant feature of polar oceans. Shifts in the distribution and extent of sea ice during the growing
season impacts the duration, magnitude, and species composition of primary and secondary production in the polar
regions. With less sea ice many marine ecosystems will experience more light, which can accelerate the growth of
phytoplankton, and shift the balance between the primary production by ice algae and water-borne phytoplankton,
with implications for Arctic food webs. In contrast, sea ice is also an important habitat for juvenile Antarctic krill,
providing food and protection from predators. Krill is a basic food source for many species in polar marine ecosystems.
Changes in sea ice will have other impacts, beyond these “bottom-up” consequences for marine food webs. Mammals
and birds utilize sea ice as haul-outs during foraging trips (seals, walrus, and polar bears in the Arctic and seals and
penguins in the Antarctic). Some seals (e.g., bearded seals in the Arctic and crab eater and leopard seals in the
Antarctic) give birth and nurse pups in pack ice. Shifts in the spatial distribution and extent of sea ice will alter the
spatial overlap of predators and their prey. According to model projections, within 50 to 70 years, loss of hunting
habitats may lead to elimination of polar bears from seasonally ice-covered areas, where two-thirds of their world
population currently live. The vulnerability of marine species to changes in sea ice will depend on the exposure to
change, which will vary by location, as well as the sensitivity of the species to changing environmental conditions
and the adaptive capacity of each species. More open waters and longer ice-free periods in the northern seas enhance
the effect of wave action and coastal erosion, with implications for coastal communities and infrastructure.
Although the overall sea ice extent in the Southern Ocean has not changed markedly in recent decades, there have
been increases in oceanic temperatures and large regional decreases in winter sea ice extent and duration in the
western Antarctic Peninsula region of West Antarctica and the islands of the Scotia Arc.

1597
Polar Regions Chapter 28
28
Amstrup, S.C., H. Caswell, E. DeWeaver, I. Stirling, D.C. Douglas, B.G. Marcot, and
C.M. Hunter, 2009: Rebuttal of “Polar bear population forecasts: a public-policy
forecasting audit”. Interfaces, 39(4), 353-369.
Amstrup, S.C., E.T. DeWeaver, D.C. Douglas, B.G. Marcot, G.M. Durner, C.M. Bitz, and
D.A. Bailey, 2010: Greenhouse gas mitigation can reduce sea-ice loss and
increase polar bear persistence. Nature, 468(7326), 955-958.
Amundsen, H., F. Berglund, and H. Westskog, 2010: Overcoming barriers to climate
change adaptation – a question of multilevel governance? Environment and
Planning C: Government and Policy,28(2), 276-289.
Andrachuk, M. and T. Pearce, 2010: Vulnerability and adaptation in two communities
in the Inuvialuit settlement region. In: Community Adaptation and Vulnerability
in Arctic Regions [Hovelsrud, G.K. and B. Smit (eds.)]. Springer, Dordrecht,
Netherlands, pp. 63-81.
Anisimov, O.A., D.G. Vaughan, T.V. Callaghan, C. Furgal, H. Marchant, T.D. Prowse, H.
Vilhjálmsson, and J.E. Walsh, 2007: Polar regions (Arctic and Antarctic). In:
Climate Change 2007: Impacts, Adaptation, and Vulnerability. Contribution of
Working Group II to the Fourth Assessment Report of the Intergovernmental
Panel on Climate Change (Parry, M.L., O.F. Canziani, J.P. Palutikof, P.J. van der
Linden, and C.E. Hanson (eds.)]. Cambridge University Press, Cambridge, UK
and New York, NY, USA, pp. 653-685.
Anisimov, O.A., E.L. Zhil’tsova, and S.A. Reneva, 2011: Estimation of critical levels of
climate change influence on the natural terrestrial ecosystems on the territory
of Russia. Meteorology and Hydrology, 36(11), 723-730.
Arctic Council, 2013:Arctic Resilience Interim Report 2013. Stockholm Environment
Institute (SEI) and Stockholm Resilience Centre, Stockholm, Sweden, 117 pp.
Armitage, D., 2008: Governance and the commons in a multi-level world. International
Journal of the Commons, 2(1), 7-32.
Armitage, J.M., C.L. Quinn, and F. Wania, 2011: Global climate change and contaminants
– an overview of opportunities and priorities for modeling the potential
implications for long-term human exposure to organic compounds in the Arctic.
Journal of Environmental Monitoring, 13(6), 1532-1546.
Armstrong, J.S., K.C. Green, and W. Soon, 2008: Polar bear population forecasts: a
public-policy forecasting audit. Interfaces, 38(5), 382-395.
Arnason, R., 2012: Global warming: new challenges for the common fisheries policy?
Ocean & Coastal Management, 70, 4-9.
Arrigo, K., G. vanDijken, and S. Bushinsky, 2008: Primary production in the Southern
Ocean, 1997-2006. Journal of Geophysical Research: Oceans, 113(C8), C08004,
doi:10.1029/2007JC004551.
Arrigo, K.R. and G.L. van Dijken, 2011: Secular trends in Arctic Ocean net primary
production. Journal of Geophysical Research: Oceans, 116(C9), C09011,
doi:10.1029/2011JC007151.
Arrigo, K.R., D.K. Perovich, R.S. Pickart, Z.W. Brown, G.L. van Dijken, K.E. Lowry, M.M.
Mills, M.A. Palmer, W.M. Balch, F. Bahr, N.R. Bates, C. Benitez-Nelson, B. Bowler,
E. Brownlee, J.K. Ehn, K.E. Frey, R. Garley, S.R. Laney, L. Lubelczyk, J. Mathis, A.
Matsuoka, B.G. Mitchell, G.W.K. Moore, E. Ortega-Retuerta, S. Pal, C.M.
Polashenski, R.A. Reynolds, B. Schieber, H.M. Sosik, M. Stephens, and J.H. Swift,
2012: Massive phytoplankton blooms under Arctic sea ice. Science, 336(6087),
1408-1408.
Astthorsson, O.S., H. Valdimarsson, A. Gudmundsdottir, and G.J. Óskarsson, 2012:
Climate-related variations in the occurrence and distribution of mackerel
(Scomber scombrus) in Icelandic waters. International Council for the
Exploration of the Sea (ICES) Journal of Marine Science, 69(7), 1289-1297.
Atkinson, A., V. Siegel, E. Pakhomov, and P. Rothery, 2004: Long-term decline in krill stock
and increase in salps within the Southern Ocean. Nature, 432(7013), 100-103.
Bajzak, C.E., M.O. Hammill, G.B. Stenson, and S. Prinsenberg, 2011: Drifting away:
implications of changes in ice conditions for a pack-ice-breeding phocid, the harp
seal (Pagophilus groenlandicus). Canadian Journal of Zoology, 89(11), 1050-1062.
Bakun, A., 2010: Linking climate to population variability in marine ecosystems
characterized by non-simple dynamics: conceptual templates and schematic
constructs. Journal of Marine Systems, 79(3-4), 361-373.
Balayla, D., T. Lauridsen, M. Søndergaard, and E. Jeppesen, 2010: Larger zooplankton
in Danish lakes after cold winters: are winter fish kills of importance?
Hydrobiologia, 646(1), 159-172.
Barber, D.G., J.V. Lukovich, J. Keogak, S. Baryluk, L. Fortier, and G.H.R. Henry, 2008:
The changing climate of the Arctic. Arctic, 61(1 Suppl.), 7-26.
Barbraud, C., C. Marteau, V. Ridoux, K. Delord, and H. Weimerskirch, 2008:
Demographic response of a population of white-chinned petrels Procellaria
aequinoctialis to climate and longline fishery bycatch. Journal of Applied
Ecology, 45(5), 1460-1467.
Barbraud, C., P. Rivalan, P. Inchausti, M. Nevoux, V. Rolland, and H. Weimerskirch,
2011: Contrasted demographic responses facing future climate change in
Southern Ocean seabirds. Journal of Animal Ecology, 80(1), 89-100.
Barbraud, C., V. Rolland, S. Jenouvrier, M. Nevoux, K. Delord, and H. Weimerskirch,
2012: Effects of climate change and fisheries bycatch on Southern Ocean
seabirds: a review. Marine Ecology Progress Series, 454, 285-307.
Barnes, D.K.A. and T. Souster, 2011: Reduced survival of Antarctic benthos linked to
climate-induced iceberg scouring. Nature Climate Change, 1(7), 365-368.
Barnes, D.K.A., D.A. Hodgson, P. Convey, C.S. Allen, and A. Clarke, 2006: Incursion
and excursion of Antarctic biota: past, present and future. Global Ecology and
Biogeography, 15(2), 121-142
Bartsch, A., T. Kumpula, B.C. Forbes, and F. Stammler, 2010: Detection of snow surface
thawing and refreezing in the Eurasian Arctic with QuikSCAT: implications for
reindeer herding. Ecological Applications, 20(8), 2346-2358.
Beaugrand, G. and R.R. Kirby, 2010: Spatial changes in the sensitivity of Atlantic
cod to climate-driven effects in the plankton. Climate Research, 41(1), 15-19.
Bednarsek, N., G.A. Tarling, D.C.E. Bakker, S. Fielding, E.M. Jones, H.J. Venables, P. Ward,
A. Kuzirian, B. Leze, R.A. Feely, and E.J. Murphy, 2012: Extensive dissolution of
live pteropods in the Southern Ocean. Nature Geoscience, 5(12), 881-885.
Beltaos, S. and T.D. Prowse, 2009: River ice hydrology in a shrinking cryosphere.
Hydrological Processes, 23(1), 122-144.
Berg, T.B., N.M. Schmidt., T.T. Høye., P.J. Aastrup., D.K. Hendrichsen., M.C. Forchhammer,
and D.R. Klein, 2008: High-Arctic plant-herbivore interactions under climate
influence. In: High-Arctic Ecosystem Dynamics in a Changing Climate [Meltofte,
H., T.R. Christensen, B. Elberling, M.C. Forchhammer, and M. Rasch (eds.)].
Advances in Ecological Research Series, Vol. 40, Elsevier Science and Technology/
Academic Press, Waltham, MA, USA, pp. 275-298.
Berkes, F., 2009: Evolution of co-management: role of knowledge generation, bridging
organizations and social learning. Journal of Environmental Management,
90(5), 1692-1702.
Berkes, F. and D. Armitage, 2010: Co-management institutions, knowledge and learning:
adapting to change in the Arctic. Etudes Inuit Studies, 34(1), 109-131.
Berkman, P.A., 2010: Environmental Security in the Arctic Ocean: Promoting Co-
operation and Preventing Conflict. Whitehall Papers (WHP) Series, 75, published
on behalf of The Royal United Services Institute for Defence and Security Studies
by Routledge Journals, Abingdon, UK, 119 pp.
Berrang-Ford, L., J.D. Ford, and J. Paterson, 2011: Are we adapting to climate change?
Global Environmental Change, 21(1), 25-33.
Bhatt, U.S., D.A. Walker, M.K. Raynolds, J.C. Comiso, H.E. Epstein, G. Jia, R. Gens, J.E.
Pinzon, C.J. Tucker, C.E. Tweedie, and P.J. Webber, 2010: Circumpolar Arctic tundra
vegetation change is linked to sea ice decline. Earth Interactions, 14(8), 1-20.
Björk, R.G. and U. Molau, 2007: Ecology of alpine snowbeds and the impact of global
change. Arctic, Antarctic, and Alpine Research, 39(1), 34-43.
Björnsson, H., T. Johannesson, and A. Snorrason, 2011: Recent climate change, projected
impacts and adaptation capacity in Iceland. In: Climate: Global Change and Local
Adaptation (Linkov, I. and T.S. Bridges (eds.)]. NATO Science for Peace and Security
Series C: Environmental Security, Springer, Dordrecht, Netherlands, pp. 465-475.
Bluhm, B.A. and R. Gradinger, 2008: Regional variability in food availability for Arctic
marine mammals. Ecological Applications, 18(2 Suppl.), S77-S96.
Bockheim, J., G. Vieira, M. Ramos, J. Lopez-Martinez, E. Serrano, M. Guglielmin, K.
Wilhelm, and A. Nieuwendam, 2013: Climate warming and permafrost dynamics
in the Antarctic Peninsula region. Global and Planetary Change, 100, 215-223.
Bogoyavlensky, D. and A. Siggner, 2004: Arctic demography. In: Arctic Human
Development Report [Einarsson, N., J.N. Larsen, A. Nilsson, and O.R. Young
(eds.)]. Stefansson Arctic Institute, Akureyri, Iceland, pp. 27-44.
Bokhorst, S.F., J.W. Bjerke, H. Tømmervik, T.V. Callaghan, and G.K. Phoenix, 2009:
Winter warming events damage sub-Arctic vegetation: consistent evidence
from an experimental manipulation and a natural event. Journal of Ecology,
97(6), 1408-1415.
Bokhorst, S., J.W. Bjerke, L.E. Street, T.V. Callaghan, and G.K. Phoenix, 2011: Impacts
of multiple extreme winter warming events on sub-Arctic heathland: phenology,
reproduction, growth, and CO
2
flux responses. Global Change Biology, 17(9),
2817-2830.
Bolton, K., M. Lougheed, J. Ford, S. Nickels, C. Grable, and J. Shirley, 2011: What We
Know, Don’t Know, and Need to Know about Climate Change in Nunavut,
Nunavik, and Nunatsiavut: A Systematic Literature Review and Gap Analysis
of the Canadian Arctic. Final report submitted to the Department of Indian and
Northern Affairs Canada, Climate Change Adaptation Program (CCAP), Inuit
Tapiriit Kanatami, Ottawa, ON, Canada, 128 pp.

1598
Chapter 28 Polar Regions
28
Borgstrøm, R. and J. Museth, 2005: Accumulated snow and summer temperature?
Critical factors for recruitment to high mountain populations of brown trout
(Salmo trutta L.). Ecology of Freshwater Fish, 14(4), 375-384.
Bouchard, C. and L. Fortier, 2011: Circum-arctic comparison of the hatching season
of polar cod Boreogadus saida: a test of the freshwater winter refuge hypothesis.
Progress in Oceanography, 90(1-4), 105-116.
Bowden, W.B., M.N. Gooseff, A. Balser, A. Green, B.J. Peterson, and J. Bradford, 2008:
Sediment and nutrient delivery from thermokarst features in the foothills of the
North Slope, Alaska: potential impacts on headwater stream ecosystems. Journal
of Geophysical Research: Biogeosciences, 113(G2), G02026, doi:10.1029/
2007JG000470.
Boyd, P.W., K.R. Arrigo, R. Strzepek, and G.L. van Dijken, 2012: Mapping phytoplankton
iron utilization: insights into Southern Ocean supply mechanisms. Journal of
Geophysical Research: Oceans, 117(C6), C06009, doi:10.1029/2011JC007726.
Brander, K.M., 2010: Cod Gadus morhua and climate change: processes, productivity
and prediction. Journal of Fish Biology, 77(8), 1899-1911.
Braun, C., O. Mustafa, A. Nordt, S. Pfeiffer, and H. Peter, 2012: Environmental
monitoring and management proposals for the Fildes Region, King George
Island, Antarctic. Polar Research, 31, 18206, doi:10.3402/polar.v31i0.18206.
Briffa, K.R., V.V. Shishov, T.M. Melvin, E.A. Vaganov, H. Grudd, R.M. Hantemirov, M.
Eronen, and M.M. Naurzbaev, 2008: Trends in recent temperature and radial
tree growth spanning 2000 years across northwest Eurasia. Philosophical
Transactions of the Royal Society B, 363(1501), 2271-2284.
Brommer, J.E., H. Pietiäinen, K. Ahola, P. Karell, T. Karstinenz, and H. Kolunen, 2010:
The return of the vole cycle in southern Finland refutes the generality of the loss
of cycles through ‘climatic forcing’. Global Change Biology, 16(2), 577-586.
Bronen, R., 2009: Forced migration of Alaskan indigenous communities due to climate
change: creating a human rights response. In: Linking Environmental Change,
Migration and Social Vulnerability [Oliver-Smith, A. and X. Shen (eds.)]. Outcomes
of the 3rd UNU-EHS Summer Academy of the Munich Re Chair on Social
Vulnerability, 27 July-2 August 2008, Hohenkammer, Germany, Studies of the
University: Research, Counsel, and Education (SOURCE) publication 12/2009,
United Nations University Institute for Environment and Human Security (UNU-
EHS), Bonn, Germany, pp. 68-73.
Bronen, R., 2011: Climate-induced community relocations: creating an adaptive
governance framework based in human rights doctrine. NYU Review of Law
and Social Change 35, 357-406.
Brown, Z.W. and K.R. Arrigo, 2013: Sea ice impacts on spring bloom dynamics and
net primary production in the eastern Bering Sea. Journal of Geophysical
Research: Oceans, 118(1), 43-62.
Brubaker, M., J. Berner, J. Bell, and J. Warren, 2011a: Climate Change in Kivalinla,
Alaska: Strategies for Community Health. Alaska Native Tribal Health Consortium
(ANTHC), Anchorage, AK, USA, 66 pp.
Brubaker, M.Y., J.N. Bell, J.E. Berner, and J.A. Warren, 2011b: Climate change health
assessment: a novel approach for Alaska Native communities. International
Journal of Circumpolar Health, 70(3), 266-273.
Brubaker, M., J. Berner, R. Chavan, and J. Warren, 2011c: Climate change and health
effects in northwest Alaska. Global Health Action, 4, 8445, doi:10.3402/
gha.v4i0.8445.
Burek, K.A., F.M.D. Gulland, and T.M. O’Hara, 2008: Effects of climate change on Arctic
marine mammal health. Ecological Applications, 18(2 Suppl.), S126-S134.
Byrd, G.V., J.A. Schmutz, and H.M. Renner, 2008: Contrasting population trends of
piscivorous seabirds in the Pribilof Islands: a 30-year perspective. Deep-Sea
Research Part II: Topical Studies in Oceanography, 55(16-17), 1846-1855.
CAFF, 2013: Arctic Biodiversity Assessment: Status and Trends in Arctic Biodiversity.
Conservation of Arctic Flora and Fauna (CAFF), Akureyri, Iceland, 557 pp.
Callaghan, T.V., L.O. Björn, Y. Chernov, F.S. Chapin III, T.R. Christensen, B. Huntley, R.
Ims, S. Jonasson, D. Jolly, N. Matveyeva, N. Panikov, W.C. Oechel, G.R. Shaver, J.
Elster, H. Henttonen, I.S. Jónsdóttir, K. Laine, S. Schaphoff, S. Sitch, E. Taulavuori,
K. Taulavuori, and C. Zöckler, 2005: Tundra and polar desert ecosystems. In:
ACIA. Arctic Climate Impacts Assessment [Symon, C., L. Arris, and B. Heal (eds.)].
Cambridge University Press, Cambridge, UK, pp. 243-352.
Callaghan, T.V., F. Bergholm, T.R. Christensen, C. Jonasson, U. Kokfelt, and M. Johansson,
2010: A new climate era in the sub-Arctic: accelerating climate changes and
multiple impacts. Geophysical Research Letters, 37(14), L14705, doi:10.1029/
2009GL042064.
Callaghan, T.V., T.R. Christensen, and E.J. Jantze, 2011a: Plant and vegetation
dynamics on Disko Island, west Greenland: snapshots separated by over 40
years. AMBIO: A Journal of the Human Environment, 40(6), 624-637.
Callaghan, T.V., C.E. Tweedie, J. Åkerman, C. Andrews, J. Bergstedt, M.G. Butler, T.R.
Christensen, D. Cooley, U. Dahlberg, R.K. Danby, F.J.A. Daniëls, J.G. de Molenaar,
J. Dick, C.E. Mortensen, D. Ebert-May, U. Emanuelsson, H. Eriksson, H. Hedenås,
G.H.R. Henry, D.S. Hik, J.E. Hobbie, E.J. Jantze, C. Jaspers, C. Johansson, M.
Johansson, D.R. Johnson, J.F. Johnstone, C. Jonasson, C. Kennedy, A.J. Kenney,
F. Keuper, S. Koh, C.J. Krebs, H. Lantuit, M.J. Lara, D. Lin, V.L. Lougheed, J. Madsen,
N. Matveyeva, D.C. McEwen, I.H. Myers-Smith, Y.K. Narozhniy, H. Olsson, V.A.
Pohjola, L.W. Price, F. Rigét, S. Rundqvist, A. Sandström, M. Tamstorf, R. Van
Bogaert, S. Villarreal, P.J. Webber, and V.A. Zemtsov, 2011b: Multi-decadal
changes in tundra environments and ecosystems: synthesis of the International
Polar Year-Back to the Future project (IPY-BTF). AMBIO: A Journal of the Human
Environment, 40(6), 705-716.
Callaghan, T.V., M. Johansson, R.D. Brown, P.Y. Groisman, N. Labba, and V. Radionov,
2011c: Changing snow cover and its impacts. In: Snow, Water, Ice and
Permafrost in the Arctic (SWIPA). Arctic Monitoring and Assessment Programme
(AMAP), Oslo, Norway, pp. 4-1 to 4-58.
Callaghan, T.V., C. Jonasson, T. Thierfelder, Z. Yang, H. Hedenås, M. Johansson, U.
Molau, R. Van Bogaert, A. Michelsen, J. Olofsson, D. Gwynn-Jones, S. Bokhorst,
G. Phoenix, J. W. Bjerke, H. Tømmervik, T.R. Christensen, E. Hanna, E. K. Koller,
and V.L. Sloan, 2013: Ecosystem change and stability over multiple decades in
the Swedish subarctic: complex processes and multiple drivers. Philosophical
Transactions of the Royal Society B, 368(1624), 20120488, doi:10.1098/
rstb.2012.0488.
Carrie, J., F. Wang, H. Sanei, R.W. Macdonald, P.M. Outridge, and G.A. Stern, 2010:
Increasing contaminant burdens in an Arctic fish, Burbot (Lota lota), in a
warming climate. Environmental Science & Technology, 44(1), 316-322.
Castro de la Guardia, L., A.E. Derocher, P.G. Myers, A.D. Terwisscha van Scheltinga,
and N.J. Lunn, 2013: Future sea ice conditions in western Hudson Bay and
consequences for polar bears in the 21
st
century. Global Change Biology, 19(9),
2675-2687.
Chapman, E.W., E.E. Hofmann, D.L. Patterson, C.A. Ribic, and W.R. Fraser, 2011:
Marine and terrestrial factors affecting Adélie penguin Pygoscelis adeliae chick
growth and recruitment off the western Antarctic Peninsula. Marine Ecology
Progress Series, 436, 273-289.
Cherosov, M.M., A.P. Isaev, V.I. Mironova, L.P. Lytkina, L.D. Gavrilyeva, R.R. Sofronov,
A.P. Arzhakova, N.V. Barashkova, I.A. Ivanov, I.F. Shurduk, A.P. Efimova, N.S.
Karpov, P.A. Timofeyev, and L.V. Kuznetsova, 2010: Vegetation and human
activity. In: The Far North: Plant Biodiversity and Ecology of Yakutia [Troeva,
E.I., A.P. Isaev, M.M. Cherosov, and N.S. Karpov (eds.)]. Springer, Berlin, Germany,
pp. 261-295.
Cherry, S.G., A.E. Derocher, I. Stirling, and E.S. Richardson, 2009: Fasting physiology
of polar bears in relation to environmental change and breeding behavior in
the Beaufort Sea. Polar Biology, 32(3), 383-391.
Cheung, W.W.L., V.W.Y. Lam, J.L. Sarmiento, K. Kearney, R. Watson, and D. Pauly, 2009:
Projecting global marine biodiversity impacts under climate change scenarios.
Fish and Fisheries, 10(3), 235-251.
Cheung, W.W.L., J. Dunne, J.L. Sarmienot, and D. Pauly, 2011: Integrating ecophysiology
and plankton dynamics into projected maximum fisheries catch potential under
climate change in the Northeast Atlantic. International Council for the
Exploration of the Sea (ICES) Journal of Marine Fisheries, 68(6), 1008-1018.
Chierici, M. and A. Fransson, 2009: Calcium carbonate saturation in the surface
water of the Arctic Ocean: undersaturation in freshwater influenced shelves.
Biogeosciences, 6(11), 2421-2431.
Chown, S.L., J.E. Lee, K.A. Hughes, J. Barmes, P.J. Barrett, D.M. Bergstrom, P. Convey,
D.A. Cowan, K. Crosbie, G. Dyer, Y. Frenot, S.M. Grant, D. Herr, M.C.I. Kennicutt, M.
Lamers, A. Murray, H.P. Possingham, K. Reid, M.J. Riddle, P.G. Ryan, L. Sanson, J.D.
Shaw, M.D. Sparrow, C. Summerhayes, A. Terauds, and D.H. Wall, 2012: Challenges
to the future conservation of the Antarctic. Science, 337(6091), 158-159.
Christoffersen, K.S., S.L. Amsinck, F. Landkildehus, T.L. Lauridsen, and E. Jeppesen,
2008: Lake flora and fauna in relation to ice-melt, water temperature and
chemistry at Zackenberg. In: High-Arctic Ecosystem Dynamics in a Changing
Climate [Meltofte, H., T.R. Christensen, B. Elberling, M.C. Forchhammer, and M.
Rasch (eds.)]. Advances in Ecological Research Series, Vol. 40, Elsevier Science
and Technology/Academic Press, Waltham, MA, USA, pp. 371-389.
Clark, D.A., D.S. Lee, M.M.R. Freeman, and S.G. Clark, 2008: Polar bear conservation
in Canada: defining the policy problems. Arctic, 61(4), 347-360.
Clarke, L.J., S.A. Robinson, Q. Hua, D.J. Ayre, and D. Fink, 2012: Radiocarbon bomb
spike reveals biological effects of Antarctic climate change. Global Change
Biology, 18(1), 301-310.

1599
Polar Regions Chapter 28
28
Cochran, P., O.H. Huntington, C. Pungowiyi, S. Tom, F.S. Chapin III, H.P. Huntington,
N.G. Maynard, and S.F. Trainor, 2013: Indigenous frameworks for observing and
responding to climate change in Alaska. Climatic Change, 12(3 SI), 557-567.
Comeau, S., R. Jeffree, J. Teyssié, and J.-P. Gattuso, 2010: Response of the Arctic
pteropod Limacina helicina to projected future environmental conditions. PLoS
ONE, 5(6), e11362, doi:10.1371/journal.pone.0011362.
Comeau, S., J.-P. Gattuso, A.M. Nisumaa, and J. Orr, 2012: Impact of aragonite
saturation state changes on migratory pteropods. Proceedings of the Royal
Society B, 279(1729), 732-738.
Constable, A.J., 2011: Lessons from CCAMLR on the implementation of the ecosystem
approach to managing fisheries. Fish and Fisheries, 12(2), 138-151.
Convey, P., 2006: Book review: Roberto Bargagli, Ecological Studies 175: Antarctic
Ecosystems – Environmental Contamination, Climate Change and Human
Impact. Journal of Paleolimnology, 36(2), 223-224.
Convey, P., 2010: Terrestrial biodiversity in Antarctica – recent advances and future
challenges. Polar Science, 4(2), 135-147.
Convey, P., 2011: Antarctic terrestrial biodiversity in a changing world. Polar Biology,
34(11), 1629-1641.
Convey, P. and M. Lebouvier, 2009: Environmental change and human impacts on
terrestrial ecosystems of the sub-Antarctic islands between their discovery and
the mid-twentieth century. Papers and Proceedings of the Royal Society of
Tasmania, 143(1), 33-44.
Convey, P. and M.I. Stevens, 2007: Antarctic biodiversity. Science, 317(5846), 1877-
1878.
Convey, P., R. Bindschadler, G. Di Prisco, E. Fahrbach, J. Gutt, D.A. Hodgson, P.A.
Mayewski, C.P. Summerhayes, J. Turner, and the ACCE Consortium, 2009:
Review: Antarctic climate change and the environment. Antarctic Science,
21(6), 541-563.
Convey, P., R. Key, M. Belchier, and C. Waller, 2011: Recent range expansions in non-
native predatory beetles on sub-Antarctic South Georgia. Polar Biology, 34(4),
597-602.
Cook, A.J., S. Poncet, A.P.R. Cooper, D.J. Herbert, and D. Christie, 2010: Glacier retreat
on South Georgia and implications for the spread of rats. Antarctic Science,
22(3), 255-263.
Cooper, L.W., C.J. Ashjian, S.L. Smith, L.A. Codispoti, J.M. Grebmeier, R.G. Campbell,
and E.B. Sherr, 2006: Rapid seasonal sea-ice retreat in the Arctic could be
affecting Pacific walrus (Odobenus rosmarus divergens) recruitment. Aquatic
Mammals, 32(1), 98-102.
Corbett, J.J., D.A. Lack, J.J. Winebrake, S. Harder, J.A. Silberman, and M. Gold, 2010:
Arctic shipping emissions inventories and future scenarios. Atmospheric
Chemistry and Physics Discussions, 10(4), 10271-10311.
Costa, D.P., L. Huckstadt, D. Crocker, B. McDonald, M. Goebel, and M. Fedak, 2010:
Approaches to studying climate change and its role on the habitat selection of
Antarctic pinnipeds. Integrative and Comparative Biology, 50(6), 1018-1030.
Cowan, D.A., S.L. Chown, P. Convey, M. Tuffin, K. Hughes, S. Pointing, and W.F. Vincent,
2011: Non-indigenous microorganisms in the Antarctic: assessing the risks.
Trends in Microbiology, 19(11), 540-548.
Coyle, K.J. and L. Van Susteren, 2012: The Psychological Effects of Global Warming
on the United States: Its Stresses, Traumas, and Societal Costs. National Wildlife
Federation (NWF), Merrifield, VA, USA, 41 pp.
Coyle, K.O., L.B. Eisner, F.J. Mueter, A.I. Pinchuk, M.A. Janout, K.D. Cieciel, E.V. Farley,
and A.G. Andrews, 2011: Climate change in the southeastern Bering Sea: impacts
on pollock stocks and implications for the oscillating control hypothesis.
Fisheries Oceanography, 20(2), 139-156.
Crate, S.A. and M. Nuttall (eds.), 2009: Anthropology and Climate Change: From
Encounters to Actions. Left Coast Press, Walnut Creek, CA, USA, 407 pp.
Crate, S.A., B.C. Forbes, L. King, and J. Kruse, 2010: Contact with nature. In: Arctic
Social Indicators: A Follow-Up to the Arctic Human Development Report [Larsen,
J.N., P. Schweitzer, and G. Fondahl (eds.)]. TemaNord 2010:519, Nordic Council
of Ministers, Copenhagen, Denmark, pp. 109-128.
Croxall, J.P., S.H.M. Butchart, B. Lascelles, A.J. Stattersfield, B. Sullivan, A. Symes, and
P. Taylor, 2012: Seabird conservation status, threats and priority actions: a global
assessment. Bird Conservation International, 22(1), 1-34.
Cruikshank, J., 2001: Glaciers and climate change: perspectives from oral tradition.
Arctic, 54(4), 377-393.
Cubillos, J.C., S.W. Wright, G. Nash, M.F. de Salas, B. Griffiths, B. Tilbrook, A. Poisson,
and G.M. Hallegraeff, 2007: Calcification morphotypes of the coccolithophorid
Emiliania huxleyi in the Southern Ocean: changes in 2001 to 2006 compared
to historical data. Marine Ecology Progress Series, 348, 47-54.
Cullen-Unsworth, L.C., R. Hill, J.R.A. Butler, and M. Wallace, 2011: A research process
for integrating indigenous and scientific knowledge in cultural landscapes:
principles and determinants of success in the Wet Tropics World Heritage Area,
Australia. The Geographical Journal, 178(4), 351-365.
Curtis, T., S. Kvernmo, and P. Bjerregaard, 2005: Changing living conditions, life style
and health. International Journal of Circumpolar Health, 64(5), 442-450.
Dalpadado, P., R.B. Ingvaldsen, L.C. Stige, B. Bogstad, T. Knutsen, G. Ottersen, and B.
Ellertsen, 2012: Climate effects on Barents Sea ecosystem dynamics. International
Council for the Exploration of the Sea (ICES) Journal of Marine Science, 69(7),
1303-1316.
Danby, R.K. and D.S. Hik, 2007: Variability, contingency and rapid change in recent
subarctic alpine tree line dynamics. Journal of Ecology, 95(2), 352-363.
Danby, R.K., S. Koh, D.S. Hik, and L.W. Price, 2011: Four decades of plant community
change in the alpine tundra of southwest Yukon, Canada. AMBIO: A Journal of
the Human Environment, 40(6), 660-671.
Daniëls, F.J.A. and J.G. De Molenaar, 2011: Flora and vegetation of Tasiilaq, formerly
Angmagssalik, Southeast Greenland: a comparison of data between around
1900 and 2007. AMBIO: A Journal of the Human Environment, 40(6), 650-659.
Dankers, R. and H. Middelkoop, 2008: River discharge and freshwater runoff to the
Barents Sea under present and future climate conditions. Climatic Change,
87(1-2), 131-153.
Dannevig, H., T. Rauken, and G. Hovelsrud, 2012: Implementing adaptation to climate
change at the local level. Local Environment: The International Journal of Justice
and Sustainability, 17(6-7), 597-611.
Darnis, G., D. Robert, C. Pomerleau, H. Link, P. Archambault, R.J. Nelson, M. Geoffroy,
J. Tremblay, C. Lovejoy, S.H. Ferguson, B.P.V. Hunt, and L. Fortier, 2012: Current
state and trends in Canadian Arctic marine ecosystems: II. Heterotrophic food
web, pelagic-benthic coupling, and biodiversity. Climatic Change, 115(1), 179-
205.
Dawson, J., E.J. Stewart, H. Lemelin, and D. Scott, 2010: The carbon cost of polar bear
viewing tourism in Churchill, Canada. Journal of Sustainable Tourism, 18(3),
319-336.
de Neergaard, E., P. Stougaard, K. Høegh, and L. Munk, 2009: Climatic changes and
agriculture in Greenland: plant diseases in potatoes and grass fields. IOP
Conference Series: Earth and Environmental Science, 6(37), 372013, doi:10.1088/
1755-1307/6/37/372013.
Degteva, A. and C. Nellemann, 2013: Nenets migration in the landscape: impacts of
industrial development in Yamal Peninsula, Russia. Pastoralism: Research, Policy
and Practice, 3, 15, doi:10.1186/2041-7136-3-15.
Derksen, C., S.L. Smith, M. Sharp, L. Brown, S. Howell, L. Copland, D.R. Mueller, Y. Gauthier,
C.G. Fletcher, A. Tivy, M. Bernier, J. Bourgeois, R. Brown, C.R. Burn, C. Duguay,
P. Kushner, A. Langlois, A.G. Lewkowicz, A. Royer, and A. Walker, 2012: Variability
and change in the Canadian cryosphere. Climatic Change, 115(1), 59-88.
Derocher, A.E., N.J. Lunn, and I. Stirling, 2004: Polar bears in a warming climate.
Integrative and Comparative Biology, 44(2), 163-176.
Derocher, A.E., M. Andersen, Ø. Wiig, J. Aars, E. Hansen, and M. Biuw, 2011: Sea ice
and polar bear den ecology at HopenIsland, Svalbard. Marine Ecology Progress
Series, 441, 273-279.
Derome, J. and N. Lukina, 2011: Interaction between environmental pollution and
land-cover/land-use change in Arctic areas. In: Eurasian Arctic Land Cover and
Land Use in a Changing Climate [Gutman, G. and A. Reissell (eds.)]. Springer,
Dordrecht, Netherlands, pp. 269-290
Déry, S.J. and E.F. Wood, 2005: Decreasing river discharge in northern Canada.
Geophysical Research Letters, 32(10), L10401, doi:10.1029/2005GL022845.
Dibike, Y., T. Prowse, T. Saloranta, and R. Ahmed, 2011: Response of Northern Hemisphere
lake-ice cover and lake-water thermal structure patterns to a changing climate.
Hydrological Processes, 25(19), 2942-2953.
Dinniman, M.S., J.M. Klinck, and E.E. Hofmann, 2012: Sensitivity of circumpolar deep
water transport and ice shelf basal melt along the West Antarctic Peninsula to
changes in the winds. Journal of Climate, 25(14), 4799-4816.
Donaldson, S.G., J. Van Oostdam, C. Tikhonov, M. Feeley, B. Armstrong, P. Ayotte, O.
Boucher, W. Bowers, L. Chan, F. Dallaire, R. Dallaire, E. Dewailly, J. Edwards, G.M.
Egeland, J. Fontaine, C. Furgal, T. Leech, E. Loring, G. Muckle, T. Nancarrow, D. Pereg,
P. Plusguellec, M. Potyrala, O. Receveur, and R.G. Shearer, 2010: Environmental
contaminants and human health in the Canadian Arctic. Science of the Total
Environment, 408(22), 5165-5234.
Donelson, J.M., P.L. Munday, M.I. Mccormick, and G.E. Nilsson, 2011: Acclimation
to predicted ocean warming through developmental plasticity in a tropical reef
fish. Global Change Biology, 17(4), 1712-1719.

1600
Chapter 28 Polar Regions
28
Drinkwater, K.F., 2011: The influence of climate variability and change on the
ecosystems of the Barents Sea and adjacent waters: review and synthesis of
recent studies from the NESSAS project. Progress in Oceanography, 90, 47-61.
Drinkwater, K.F., G. Beaugrand, M. Kaeriyama, S. Kim, G. Ottersen, R.I. Perry, H.-O.
Pörtner, J.J. Polovina, and A. Takasuka, 2010: On the processes linking climate
to ecosystem changes. Journal of Marine Systems, 79(3-4), 374-388.
Duc, N., P. Crill, and D. Bastviken, 2010: Implications of temperature and sediment
characteristics on methane formation and oxidation in lake sediments.
Biogeochemistry, 100(1), 185-196.
Ducklow, H., A. Clarke, R. Dickhut, S.C. Doney, H. Geisz, K. Huang, D.G. Martinson,
M.P. Meredith, H.V. Moeller, M. Montes-Hugo, O. Schofield, S.E. Stammerjohn,
D. Steinberg, and W. Fraser, 2012: The marine system of the western Antarctic
Peninsula. In: Antarctic Ecosystems: An Extreme Evironment in a Changing
World [Rogers, A.D., N.M. Johnston, E.J. Murphy, and A. Clarke (eds.)]. John
Wiley & Sons, Ltd., Chichester, UK, pp. 121-159.
Duhaime, G., A. Lemelin, V. Didyk, O. Goldsmith, G. Winther, A. Caron, N. Bernard,
and A. Godmaire, 2004: Economic systems. In: The Arctic Human Development
Report [Einarsson, N., J.N. Larsen, A. Nilsson, and O.R. Young (eds.)]. Stefansson
Arctic Institute, Akureyri, Iceland, pp. 69-84.
Dumas, J.A., G.M. Flato, and R.D. Brown, 2006: Future projections of landfast ice
thickness and duration in the Canadian Arctic. Journal of Climate, 19(20),
5175-5189.
Durner, G.M., D.C. Douglas, R.M. Nielson, S.C. Amstrup, T.L. McDonald, I. Stirling, M.
Mauritzen, E.W. Born, Ø. Wiig, E. DeWeaver, M.C. Serreze, S.E. Belikov, M.M.
Holland, J. Maslanik, J. Aars, D.A. Bailey, and A.E. Derocher, 2009: Predicting
21
s
t
-century polar bear habitat distribution from global climate models.
Ecological Monographs, 79(1), 25-58.
Durner, G., J. Whiteman, H. Harlow, S. Amstrup, E. Regehr, and M. Ben-David, 2011:
Consequences of long-distance swimming and travel over deep-water pack ice
for a female polar bear during a year of extreme sea ice retreat. Polar Biology,
34(7), 975-984.
Dyck, M.G. and E. Kebreab, 2009: Estimating the energetic contribution of polar bear
(Ursus Maritimus) summer diets to the total energy budget. Journal of
Mammalogy, 90(3), 585-593.
Dyck, M. and S. Romberg, 2007: Observations of a wild polar bear (Ursus maritimus)
successfully fishing Arctic charr (Salvelinus alpinus) and Fourhorn sculpin
(Myoxocephalus quadricornis). Polar Biology, 30(12), 1625-1628.
Dyck, M.G., W. Soon, R.K. Baydack, D.R. Legates, S. Baliunas, T.F. Ball, and L.O.
Hancock, 2007: Polar bears of western Hudson Bay and climate change: are
warming spring air temperatures the “ultimate” survival control factor?
Ecological Complexity, 4(3), 73-84.
Dyck, M.G., W. Soon, R.K. Baydack, D.R. Legates, S. Baliunas, T.F. Ball, and L.O.
Hancock, 2008: Reply to response to Dyck et al. (2007) on polar bears and
climate change in western Hudson Bay by Stirling et al. (2008). Ecological
Complexity, 5(4), 289-302.
Eicken, H., A.L. Lovecraft, and M.L. Druckenmiller, 2009: Sea-ice system services: a
framework to help identify and meet information needs relevant for observing
networks. Arctic, 62(2), 119-136.
Eira, I.M.G., C. Jaedicke, O.H. Magga, N.G. Maynard, D. Vikhamar-Schuler, and S.D.
Mathiesen, 2012: Traditional Sámi snow terminology and physical snow
classification – two ways of knowing. Cold Regions Science and Technology,
85, 117-130.
Ellingsen, I.H., P. Dalpadado, D. Slagstad, and H. Loeng, 2008: Impact of climatic
change on the biological production in the Barents Sea. Climatic Change,
87(1-2), 155-175.
Elmendorf, S.C., G.H.R. Henry, R.D. Hollister, R.G. Björk, A.D. Bjorkman, T.V. Callaghan,
L.S. Collier, E.J. Cooper, J.H.C. Cornelissen, T.A. Day, A.M. Fosaa, W.A. Gould, J.
Grétarsdóttir, J. Harte, L. Hermanutz, D.S. Hik, A. Hofgaard, F. Jarrad, I.S.
Jónsdóttir, F. Keuper, K. Klanderud, J.A. Klein, S. Koh, G. Kudo, S.I. Lang, V.
Loewen, J.L. May, J. Mercado, A. Michelsen, U. Molau, I.H. Myers-Smith, S.F.
Oberbauer, S. Pieper, E. Post, C. Rixen, C.H. Robinson, N.M. Schmidt, G.R. Shaver,
A. Stenström, A. Tolvanen, Ø. Totland, T. Troxler, C.-H. Wahren, P.J. Webber, J.M.
Welker, and P.A. Wookey, 2012a: Global assessment of experimental climate
warming on tundra vegetation: heterogeneity over space and time. Ecology
Letters, 15(2), 164-175.
Elmendorf, S.C., G.H.R. Henry, R.D. Hollister, R.G. Björk, N. Boulanger-Lapointe, E.J.
Cooper, J.H.C. Cornelissen, T.A. Day, E. Dorrepaal, T.G. Elumeeva, M. Gill, W.A.
Gould, J. Harte, D.S. Hik, A. Hofgaard, D.R. Johnson, J.F. Johnstone, I.S. Jónsdóttir,
J.C. Jorgenson, K. Klanderud, J.A. Klein, S. Koh, G. Kudo, M. Lara, E. Lévesque,
B. Magnússon, J.L. May, J.A. Mercado-Díaz, A. Michelsen, U. Molau, I.H. Myers-
Smith, S.F. Oberbauer, V.G. Onipchenko, C. Rixen, N.M. Schmidt, G.R. Shaver,
M.J. Spasojevic, Þ.E. Þórhallsdóttir, A. Tolvanen, T. Troxler, C.E. Tweedie, S.
Villareal, C.-H. Wahren, X. Walker, P.J. Webber, J.M. Welker, and S. Wipf, 2012b:
Plot-scale evidence of tundra vegetation change and links to recent summer
warming. Nature Climate Change, 2, 453-457.
Emmerton, C.A., L.F.W. Lesack, and W.F. Vincent, 2008: Mackenzie River nutrient
delivery to the Arctic Ocean and effects of the Mackenzie Delta during open
water conditions. Global Biogeochemical Cycles, 22(1), GB1024, doi:10.1029/
2006GB002856.
Epstein, H.E., M.K. Raynolds, D.A. Walker, U.S. Bhatt, C.J. Tucker, and J.E. Pinzon, 2012:
Dynamics of aboveground phytomass of the circumpolar Arctic tundra during the
past three decades. Environmental Research Letters, 7(1), 015506, doi:10.1088/
1748-9326/7/1/015506.
Epstein, P.R. and D. Ferber, 2011: Changing Planet, Changing Health: How the Climate
Crisis Threatens Our Health and What We Can Do about It. University of
California Press, Berkeley and Los Angeles, CA, USA, 368 pp.
Eskeland, G.S. and L.S. Flottorp, 2006: Climate change in the Arctic: a discussion of
the impact on economic activity. In: The Economy of the North [Glomsrød, S.
and I. Aslaksen (eds.)].Statistics Norway,Oslo, Norway, pp. 81-94.
Fabry, V.J., J.B. McClintock, J.T. Mathis, and J.M. Grebmeier, 2009: Ocean acidification
at high latitudes: the bellwether. Oceanography, 22(4), 160-171.
Falk-Petersen, S., P. Mayzaud, G. Kattner, and J. R. Sargent. 2009: Lipids and life
strategy of Arctic Calanus. Marine Biology Research, 5(1 SI), 18-39.
Favero-Longo, S.E., N. Cannone, M.R. Worland, P. Convey, R. Piervittori, and M.
Guglielmin, 2011: Changes in lichen diversity and community structure with
fur seal population increase on Signy Island, South Orkney Islands. Antarctic
Science, 23(1), 65-77.
Fechhelm, R.G., B. Streever, and B.J. Gallaway, 2007: The arctic cisco (Coreogonus
autumnalis) subsistence and commercial fisheries, Colville River, Alaska: a
conceptual model.Arctic, 60(4), 421-429.
Ferguson, S.H., I. Stirling, and P. McLoughlin, 2005: Climate change and ringed seal
(Phoca hispida) recruitment in western Hudson Bay. Marine Mammal Science,
21(1), 121-135.
Ferguson, S.H., M. Kingsley, and J. Higdon, 2012: Killer whale (Orcinus orca)
predation in a multi-prey system. Population Ecology, 54(1), 31-41.
Fischbach, A.S., S.C. Amstrup, and D.C. Douglas, 2007: Landward and eastward shift
of Alaskan polar bear denning associated with recent sea ice changes. Polar
Biology, 30(11), 1395-1405.
Flanagan, K.M., E. McCauley, and F. Wrona, 2006: Freshwater food webs control
carbon dioxide saturation through sedimentation. Global Change Biology,
12(4), 644-651.
Flores, H., A. Atkinson, S. Kawaguchi, B.A. Krafft, G. Milinevsky, S. Nicol, C. Reiss, G.A.
Tarling, R. Werner, E.B. Rebolledo, V. Cirelli, J. Cuzin-Roudy, S. Fielding, J.J.
Groeneveld, M. Haraldsson, A. Lombana, E. Marschoff, B. Meyer, E.A. Pakhomov,
E. Rombolá, K. Schmidt, V. Siegel, M. Teschke, H. Tonkes, J.Y. Toullec, P.N. Trathan,
N. Tremblay, A.P. Van de Putte, J.A. van Franeker, and T. Werner, 2012: Impact of
climate change on Antarctic krill. Marine Ecology Progress Series, 458, 1-19.
Forbes, B.C., 2006: The challenges of modernity for reindeer management in
northernmost Europe. In: Reindeer Management in Northernmost Europe: Linking
Practical and Scientific Knowledge in Social-Ecological Systems [Forbes, B.C.,
M. Bölter, L. Müller-Wille, J. Hukkinen, F. Müller, N. Gunslay, and Y. Konstantinov
(eds.)]. Ecological Studies, Vol. 184, Springer-Verlag, Berlin, Germany, pp. 11-25.
Forbes, B.C., 2008: Equity, vulnerability and resilience in social-ecological systems:
a contemporary example from the Russian Arctic. Research in Social Problems
and Public Policy, 15, 203-236.
Forbes, B.C. and T. Kumpula, 2009: The ecological role and geography of reindeer
(Rangifer tarandus) in northern Eurasia. Geography Compass, 3(4), 1356-1380.
Forbes, B.C. and F. Stammler, 2009: Arctic climate change discourse: the contrasting
politics of research agendas in the West and Russia. Polar Research, 28(1), 28-
42.
Forbes, B.C., M. Bölter, L. Müller-Wille, J. Hukkinen, F. Müller, N. Gunslay, and Y.
Konstantinov (eds.), 2006: Reindeer Management in Northernmost Europe:
Linking Practical and Scientific Knowledge in Social-Ecological Systems.
Ecological Studies, Vol. 184, Springer, Berlin, Germany, 397 pp.
Forbes, B.C., F. Stammler, T. Kumpula, N. Meschtyb, A. Pajunen, and E. Kaarlejärvi,
2009: High resilience in the Yamal-Nenets social-ecological system, West
Siberian Arctic, Russia. Proceedings of the National Academy of Sciences of the
United States of America, 106(52), 22041-22048.

1601
Polar Regions Chapter 28
28
Forbes, B.C., M.M. Fauria, and P. Zetterberg, 2010: Russian Arctic warming and
‘greening’ are closely tracked by tundra shrub willows. Global Change Biology,
16(5), 1542-1554.
Forbes, D.L. (ed.), 2011: State of the Arctic Coast 2010: Scientific Review and Outlook.
International Arctic Science Committee, Land-Ocean Interactions in the Coastal
Zone, Arctic Monitoring and Assessment Programme, International Permafrost
Association, Helmholtz-Zentrum, Geesthacht, Germany,178 pp.
Forcada, J., P.N. Trathan, P.L. Boveng, I.L. Boyd, J.M. Burns, D.P. Costa, M. Fedak, T.L.
Rogers, and C.J. Southwell, 2012: Responses of Antarctic pack-ice seals to
environmental change and increasing krill fishing. Biological Conservation,
149(1), 40-50.
Forchhammer, M.C., N.M. Schmidt, T.T. Høye, T.B. Berg, D.K. Hendrichsen, and E. Post,
2008: Population dynamical responses to climate change. In: High-Arctic
Ecosystem Dynamics in a Changing Climate [Meltofte, H., T.R. Christensen, B.
Elberling, M.C. Forchhammer, and M. Rasch (eds.)]. Advances in Ecological
Research Series, Vol. 40, Elsevier Science and Technology/Academic Press,
Waltham, MA, USA, pp. 391-419.
Ford, J.D., 2009: Dangerous climate change and the importance of adaptation for
the Arctic’s Inuit population. Environmental Research Letters, 4(2), 024006,
doi:10.1088/1748-9326/4/2/024006.
Ford, J.D. and L. Berrang-Ford, 2009: Food security in Igloolik, Nunavut: an exploratory
study. Polar Record, 45(3), 225-236.
Ford, J.D. and C. Furgal, 2009: Foreword to the special issue: climate change impacts,
adaptation and vulnerability in the Arctic. Polar Research, 28(1 SI), 1-9.
Ford, J.D. and C. Goldhar, 2012: Climate change vulnerability and adaptation in
resource dependent communities: a case study from West Greenland. Climate
Research, 54, 181-196.
Ford, J.D. and T. Pearce, 2010: What we know, do not know, and need to know about
climate change vulnerability in the western Canadian Arctic: a systematic
literature review. Environmental Research Letters, 5(1), 014008, doi:10.1088/
1748-9326/5/1/014008.
Ford, J.D., B. Smit, and J. Wandel, 2006: Vulnerability to climate change in the Arctic:
case study from Arctic Bay, Nunavut. Global Environmental Change, 16(2), 145-
160.
Ford, J., B. Pearce, B. Smit, J. Wandel, M. Allurut, K. Shappa, H. Ittusujurat, and K.
Qrunnut, 2007: Reducing vulnerability to climate change in the Arctic: the case
of Nunavut, Canada. Arctic, 60(2), 150-166.
Ford, J., T. Pearce, J. Prno, F. Duerden, L. Berrang Ford, M. Beaumier, and T. Smith, 2010:
Perceptions of climate change risks in primary resource use industries: survey
of the Canadian mining sector. Regional Environmental Change, 10(1), 65-81.
Frederiksen, M., T. Anker-Nilssen, G. Beaugrand, and S. Wanless, 2013: Climate,
copepods and seabirds in the boreal Northeast Atlantic – current state and
future outlook. Global Change Biology, 19(2), 364-372.
Freitas, C., K.M. Kovacs, R.A. Ims, and C. Lydersen, 2008: Predicting habitat use
by ringed seals (Phoca hispida) in a warming Arctic. Ecological Modelling,
217(1-2), 19-32.
Frenot, Y., S.L. Chown, J. Whinam, P.M. Selkirk, P. Convey, M. Skotnicki, and D.M.
Bergstrom, 2005: Biological invasions in the Antarctic: extent, impacts and
implications. Biological Reviews, 80(1), 45-72.
Frey, K.E. and J.W. McClelland, 2009: Impacts of permafrost degradation on arctic
river biogeochemistry. Hydrological Processes, 23(1), 169-182.
Froese, R. and A. Proelß, 2010: Rebuilding fish stocks no later than 2015: will Europe
meet the deadline? Fish and Fisheries, 11(2), 194-202.
Fuglei, E. and R.A. Ims, 2008: Global warming and effects on the arctic fox. Science
Progress, 91(2), 175-191.
Furgal, C., 2008: Climate change health vulnerabilities in the North. In: Human Health
in a Changing Climate: a Canadian Assessment of Vulnerabilities and Adaptive
Capacity [Seguin, J. (ed.)]. Health Canada, Ottawa, ON, Canada, pp. 303-366.
Furgal, C. and T.D. Prowse, 2008: Northern Canada. In: From Impacts to Adaptation:
Canada in a Changing Climate [Lemmen, D.S., F.J. Warren, and J. Lacroix (ed.)].
Climate Change Impacts and Adaptation Division, Natural Resources Canada,
Ottawa, ON, Canada, pp. 57-118
Furgal, C. and J. Seguin, 2006: Climate change, health, and vulnerability in Canadian
northern aboriginal communities. Environmental Health Perspectives, 114(12),
1964-1970.
Galand, P.E., C. Lovejoy, J. Pouliot, M.E. Garneau, and W.F. Vincent, 2008: Microbial
community diversity and heterotrophic production in a coastal Arctic ecosystem:
a stamukhi lake and its source waters. Limnology and Oceanography, 53(2),
813-823.
Galloway-McLean, K., 2010: Advance Guard: Climate Change Impacts, Adaptation,
Mitigation and Indigenous Peoples – A Compendium of Case Studies. United
Nations University, Institute for Advanced Studies (UNU-IAS), Traditional
Knowledge Initiative, Darwin, NT, Australia, 124 pp.
Gamon, J.A., K.F. Heummrich, R.S. Stone, and C.E. Tweedie, 2013: Spatial and
temporal variation in primary productivity (NDVI) of coastal Alaskan tundra:
decreased vegetation growth following earlier snowmelt. Remote Sensing of
Environment, 129, 144-153.
GAO, 2009: Alaska Native Villages: Limited Progress Has Been Made on Relocating
Villages Threatened by Flooding and Erosion. Report to Congressional
Requesters, GAO-09-551, United States Government Accountability Department
(GAO), Washington, DC, USA, 49 pp.
Gaston, A.J., 2011: Arctic seabirds: diversity, populations, trends, and causes. In:
Gyrfalcons and Ptarmigan in a Changing World, Vol. I [Watson, R.T., T. J. Cade,
M. Fuller, G. Hunt, and E. Potapov (eds.)]. The Peregrine Fund, Boise, ID, USA,
pp. 147-160.
Gaston, A.J. and K. Woo, 2008: Razorbills (Alca torda) follow subarctic prey into the
Canadian Arctic: colonization results from climate change? The Auk, 125(4),
939-942.
Gaston, A.J., H.G. Gilchrist, and J.M. Hipfner, 2005: Climate change, ice conditions
and reproduction in an Arctic nesting marine bird: Brunnich’s guillemot (Uria
lomvia L.). Journal of Animal Ecology, 74(5), 832-841.
Gaston, A.J., H.G. Gilchrist, M.L. Mallory, and P.A. Smith, 2009: Changes in seasonal
events, peak food availability, and consequent breeding adjustment in a marine
bird: a case of progressive mismatching. Condor, 111(1), 111-119.
Gautier, D.L., K.J. Bird, R.R. Charpentier, A. Grantz, D.W. Houseknecht, T.R. Klett, T.E.
Moore, J.K. Pitman, C.J. Schenk, J.H. Schuenemeyer, K. Sørensen, M.E. Tennyson,
Z.C. Valin, and C.J. Wandrey, 2009: Assessment of undiscovered oil and gas in
the Arctic. Science, 324(5931), 1175-1179.
Gearheard, S., W. Matumeak, I. Angutikjuaq, J. Maslanik, H.P. Huntington, J. Leavitt,
D.M. Kagak, G. Tigullaraq, and R.G. Barry, 2006: “It’s not that simple”: a
collaborative comparison of sea ice environments, their uses, observed changes,
and adaptations in Barrow, Alaska, USA, and Clyde River, Nunavut, Canada.
AMBIO: A Journal of the Human Environment, 35(4), 203-211.
Gearheard, S., G. Aipellee, and K. O’Keefe, 2010: The Igliniit project: combining Inuit
knowledge and geomatics engineering to develop a new observation tool for
hunters. In: SIKU: Knowing Our Ice, Documenting Inuit Sea-Ice Knowledge and
Use [Krupnik, I., C. Aporta, S. Gearheard, G.J. Laidler, and L. Kielsen-Holm (eds.)].
Springer, Dordrecht, Netherlands, pp. 181-202.
Gearheard, S., C. Aporta, G. Aipellee, and K. O’Keefe, 2011: The Igliniit project: Inuit
hunters document life on the trail to map and monitor arctic change. Canadian
Geographer / Le Géographe Canadien, 55(1), 42-55.
Geffen, A.J., H. Høie, A. Folkvord, A.K. Hufthammer, C. Andersson, U. Ninnemann, R.B.
Pedersen, and K. Nedreaas, 2011: High-latitude climate variability and its effect
on fisheries resources as revealed by fossil cod otoliths. International Council
for the Exploration of the Sea (ICES) Journal of Marine Science, 68(6), 1081-
1089.
Gilg, O., B. Sittler, and I. Hanski, 2009: Climate change and cyclic predator-prey population
dynamics in the High Arctic. Global Change Biology, 15(11), 2634-2652.
Gilg, O., K.M. Kovacs, J. Aars, J. Fort, G. Gauthier, D. Grémillet, R.A. Ims, H. Meltofte,
J. Moreau, E. Post, N.M. Schmidt, G. Yannic, and L. Bollache, 2012: Climate
change and the ecology and evolution of Arctic vertebrates. Annals of the New
York Academy of Sciences, 1249, 166-190.
Gjosaeter, H., B. Bogstad, and S. Tjelmeland, 2009: Ecosystem effects of the three
capelin stock collapses in the Barent Sea. Marine Biology Research, 5(1 SI),
40-53.
Gleason, J.S. and K.D. Rode, 2009: Polar bear distribution and habitat association
reflect long-term changes in fall sea ice conditions in the Alaskan Beaufort Sea.
Arctic, 62(4), 405-417.
Glomsrød, S. and I. Aslaksen, 2009: The Economy of the North 2008. Statistics
Norway, Oslo, Norway, 102 pp.
Goetz, S.J., A.G. Bunn, G.J. Fiske, and R.A. Houghton, 2005: Satellite-observed
photosynthetic trends across boreal North America associated with climate and
fire disturbance. Proceedings of the National Academy of Sciences of the United
States of America, 102(38), 13521-13525.
Grant, S., A. Constable, B. Raymond, and S. Doust, 2006: Bioregionalisation of the
Southern Ocean: Report of Experts Workshop, Hobart, September 2006. World
Wide Fund for Nature – Australia (WWF-Australia) and the Antarctic Climate and
Ecosystems Cooperative Research Center (ACE CRC), Sydney, Australia, 45 pp.

1602
Chapter 28 Polar Regions
28
Grebmeier, J.M., 2012: Shifting patterns of life in the Pacific Arctic and sub-Arctic
seas. Annual Review of Marine Science, 4(1), 63-78.
Grebmeier, J.M., J.E. Overland, S.E. Moore, E.V. Farley, E.C. Carmack, L.W. Cooper,
K.E. Frey, J.H. Helle, F.A. McLaughlin, and S.L. McNutt, 2006: A major ecosystem
shift in the northern Bering Sea. Science, 311(5766), 1461-1464.
Green, D. and G. Raygorodetsky, 2010: Indigenous knowledge of a changing climate.
Climatic Change, 100(2), 239-242.
Greenwald, M.J., W.B. Bowden, M.N. Gooseff, J.P. Zarnetske, J.P. McNamara, J.H.
Bradford, and T.R. Brosten, 2008: Hyporheic exchange and water chemistry of
two arctic tundra streams of contrasting geomorphology. Journal of Geophysical
Research, 113, G02029, doi:10.1029/2007JG000549.
Grémillet, D. and T. Boulinier, 2009: Spatial ecology and conservation of seabirds facing
global climate change: a review. Marine Ecology Progress Series, 391, 121-137.
Grémillet, D., J. Welcker, N.J. Karnovsky, W. Walkusz, M.E. Hall, J. Fort, Z.W. Brown,
J.R. Speakman, and A.M.A. Harding, 2012: Little auks buffer impacts of current
Arctic climate change. Marine Ecology Progress Series, 45, 197-206.
Grenfell, T.C. and J. Putkonen, 2008: A method for the detection of the severe
rain-on-snow event on Banks Island, October 2003, using passive microwave
remote sensing. Water Resources Research, 44(3), W03425, doi:10.1029/
2007WR005929.
Griffith, B., L.G. Adams, D.C. Douglas, C. Cuyler, R.G. White, A. Gunn, D.E. Russell, and
R.D. Cameron, 2010: No evidence of trophic mismatch for caribou in Greenland.
Science, E-Letter published online 13 January 2010, www.sciencemag.org/
content/325/5946/1355/reply#sci_el_12934.
Grønlund, A., 2009: Virkning av Klimaendring på Arealbruk i Norsk Arktis. Bioforsk
Rapport,Vol. 4, No.109, Bioforsk, Norwegian Institute for Agricultural and
Environmental Research, Ås, Norway, 15 pp. (in Norwegian).
Gudasz, C., D. Bastviken, K. Steger, K. Premke, S. Sobek, and L.J. Tranvik, 2010:
Temperature-controlled organic carbon mineralization in lake sediments.
Nature, 466(7305), 478-481.
Gunn, A., F.L. Miller, S.J. Barry, and A. Buchan, 2009: A near-total decline in caribou
on Prince of Wales, Somerset, and Russell Islands, Canadian Arctic. Arctic, 59(1),
1-13.
Gutt, J., I. Barratt, E. Domack, C.D. d’Acoz, W. Dimmler, A. Grémare, O. Heilmayer, E.
Isla, D. Janussen, E. Jorgensen, K.H. Kock, L.S. Lehnert, P. López-Gonzáles, S.
Langner, K. Linse, M.E. Manjón-Cabeza, M. Meißner, A. Montiel, M. Raes, H.
Robert, A. Rose, E.S. Schepisi, T. Saucède, M. Scheidat, H.-W. Schenke, J. Seiler,
and C. Smith, 2011: Biodiversity change after climate-induced ice-shelf collapse
in the Antarctic. Deep-Sea Research Part II: Topical Studies in Oceanography,
58(1-2), 74-83.
Halldórsson, G., B.D. Sigurdsson, O.E.S. Hrafnkelsdóttir, Ó. Eggertsson, and E. Ólafsson,
2013: New anthropod herbivores on trees and shrubs in Iceland and changes
in pest dynamics: a review. Icelandic Agricultural Sciences, 26, 69-84.
Hallinger, M., M. Manthey, and M. Wilmking, 2010: Establishing a missing link: warm
summers and winter snow cover promote shrub expansion into alpine tundra
in Scandinavia. New Phytologist, 186(4), 890-899.
Hansen, B.B., R. Aanes, I. Herfindal, J. Kohler, B.-E. Sæther, and M.K. Oli, 2011: Climate,
icing, and wild arctic reindeer: past relationships and future prospects. Ecology,
92(10), 1917-1923.
Hansen, B.B., V. Grøtan, R. Aanes, B.-E. Sæther, A. Stien, E. Fuglei, R.A. Ims, N.G. Yoccoz,
and A.Ø. Pedersen, 2013: Climate events synchronize the dynamics of a resident
vertebrate community in the High Arctic. Science, 339(6117), 313-315.
Harsch, M.A., P.E. Hulme, M.S. McGlone, and R.P. Duncan, 2009: Are treelines
advancing? A global meta-analysis of treeline response to climate warming.
Ecology Letters, 12(10), 1040-1049.
Harsem, Ø., A. Eide, and K. Heen, 2011: Factors influencing future oil and gas
prospects in the Arctic. Energy Policy, 39(12), 8037-8045.
Hátún, H., M.R. Payne, and J.A. Jacobsen, 2009: The North Atlantic subpolar gyre
regulates the spawning distribution of blue whiting (Micromesistius poutassou).
Canadian Journal of Fisheries and Aquatic Sciences, 66(5), 759-770.
Hay, L. and G. McCabe, 2010: Hydrologic effects of climate change in the Yukon River
Basin. Climatic Change, 100(3-4), 509-523.
Haynie, A.C. and L. Pfeiffer, 2012: Why economics matters for understanding the effects
of climate change on fisheries. International Council for the Exploration of the
Sea (ICES) Journal of Marine Science, 69(7), 1160-1167.
Hedenås, H., H. Olsson, C. Jonasson, J. Bergstedt, U. Dahlberg, and T.V. Callaghan,
2011: Changes in tree growth, biomass and vegetation over a 13-year period
in the Swedish sub-Arctic. AMBIO: A Journal of the Human Environment, 40(6),
672-682.
Hedensted, D.L., K. Sehested, T. Hellesen, and V. Nellemann, 2012: Climate change
adaptation in Denmark: enhancement through collaboration and meta-
governance? Local Environment, 17(6-7 SI), 613-628.
Heggberget, T.M., E. Gaare, and J.P. Ball, 2010: Reindeer (Rangifer tarandus) and
climate change: importance of winter forage. Rangifer, 22(1), 13-31.
Heino, J., R. Virkkala, and H. Toivonen, 2009: Climate change and freshwater
biodiversity: detected patterns, future trends and adaptations in northern
regions. Biological Reviews, 84(1), 39-54.
Heliasz, M., T. Johansson, A. Lindroth, M. Mölder, M. Mastepanov, T. Friborg, T.V.
Callaghan, and T.R. Christensen, 2011: Quantification of C uptake in subarctic
birch forest after setback by an extreme insect outbreak. Geophysical Research
Letters, 38(1), L01704, doi:10.1029/2010GL044733.
Helle, T.P. and L.M. Jaakkola, 2008: Transitions in management of semi-domesticated
reindeer in northern Finland. Annales Zoologici Fennici, 45(2), 81-101.
Henden, J.-A., R.A. Ims, N.G. Yoccoz, P. Hellström, and A. Angerbjörn, 2010: Strength
of asymmetric competition between predators in food webs ruled by fluctuating
prey: the case of foxes in tundra. Oikos, 119(1), 27-34.
Hezel, P.J., X. Zhang, C.M. Bitz, B.P. Kelly, and F. Massonnet, 2012: Projected decline
in spring snow depth on Arctic sea ice caused by progressively later autumn
open ocean freeze-up this century. Geophysical Research Letters, 39(17),
L17505, doi:10.1029/2012GL052794.
Higdon, J.W. and S.H. Ferguson, 2009: Loss of Arctic sea ice causing punctuated
change in sightings of killer whales (Orcinus orca) over the past century.
Ecological Applications, 19(5), 1365-1375.
Hill, G.B. and G.H.R. Henry, 2011: Responses of High Arctic wet sedge tundra to
climate warming since 1980. Global Change Biology, 17(1), 276-287.
Hill, S.L., T. Phillips, and A. Atkinson, 2013: Potential climate change effects on the
habitat of Antarctic krill in the Weddell Quadrant of the Southern Ocean. PLoS
ONE, 8(8), e72246, doi:10.1371/journal.pone.0072246.
Hinkel, K.M., B.M. Jones, W.R. Eisner, C.J. Cuomo, R.A. Beck, and R. Frohn, 2007:
Methods to assess natural and anthropogenic thaw lake drainage on the western
Arctic coastal plain of northern Alaska. Journal of Geophysical Research: Earth
Surface, 112(F2), F02S16, doi:10.1029/2006JF000584.
Hinz, D.J., A.J. Poulton, M.C. Nielsdóttir, S. Steigenberger, R.E. Korb, E.P. Achterberg,
and T.S. Bibby, 2012: Comparative seasonal biogeography of mineralising
nannoplankton in the Scotia Sea: Emiliania huxleyi, Fragilariopsis spp. and
Tetraparma pelagica. Deep-Sea Research Part II: Topical Studies in Oceanography,
59-60, 57-66.
Hinzman, L.D., N.D. Bettez, W.R. Bolton, F.S. Chapin III, M.B. Dyurgerov, C.L. Fastie, B.
Griffith, R.D. Hollister, A. Hope, H.P. Huntington, A.M. Jensen, G.J. Jia, T. Jorgenson,
D.L. Kane, D.R. Klein, G. Kofinas, A.H. Lynch, A.H. Lloyd, A.D. McGuire, F.E. Nelson,
W.C.Oechel, T.E. Osterkamp, C.H. Racine, V.E. Romanovsky, R.S. Stone, D.A. Stow,
M. Sturm, C.E. Tweedie, G.L. Vourlitis, M.D. Walker, D.A. Walker, P.J. Webber, J.M.
Welker, K.S. Winker, and K. Yoshikawa, 2005: Evidence and implications of recent
climate change in northern Alaska and other arctic regions. Climatic Change,
72(3), 251-298.
Hodgson, D.A., 2011: First synchronous retreat of ice shelves marks a new phase of
polar deglaciation. Proceedings of the National Academy of Sciences of the
United States of America, 108(47), 18859-18860.
Hodgson, D.A. and J.P. Smol, 2008: High latitude paleolimnology. In: Polar Lakes and
Rivers – Limnology of Arctic and Antarctic Aquatic Ecosystems [Vincent, W.F.
and J. Laybourn-Parry (eds.)]. Oxford University Press, Oxford, UK, pp. 43-64.
Hodgson, D.A., E. Verleyen, K. Sabbe, A.H. Squier, B.J. Keely, M.J. Leng, K.M. Saunders,
and W. Vyverman, 2005: Late Quaternary climate-driven environmental change
in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment
core. Quaternary Research, 64(1), 83-99.
Hodgson, D.A., D. Roberts, A. McMinn, E. Verleyen, B. Terry, C. Corbett, and W. Vyverman,
2006a: Recent rapid salinity rise in three East Antarctic lakes. Journal of
Paleolimnology, 36, 385-406.
Hodgson, D.A., E. Verleyen, A.H. Squier, K. Sabbe, B.J. Keely, K.M. Saunders, and W.
Vyverman, 2006b: Interglacial environments of coastal east Antarctica:
comparison of MIS 1 (Holocene) and MIS 5e (Last Interglacial) lake-sediment
records. Quaternary Science Reviews, 25, 179-197.
Hofgaard, A., J.O. Lokken, L. Dalen, and H. Hytteborn, 2010: Comparing warming
and grazing effects on birch growth in an alpine environment – a 10-year
experiment. Plant Ecology and Diversity, 3(1), 19-27.
Hollowed, A.B., B. Planque, and H. Loeng, 2013: Potential movement of fish and
shellfish stocks from the sub-Arctic to the Arctic Ocean. Fisheries Oceanography,
22(5), 355-370.

1603
Polar Regions Chapter 28
28
Hovelsrud, G.K. and B. Smit (eds.), 2010: Community Adaptation and Vulnerability
in Arctic Regions. Springer, Dordrech, Netherlands, 353 pp.
Hovelsrud, G.K., B. Poppel, B.E.H. van Oort, and J.D. Reist, 2011: Arctic societies,
cultures, and peoples. In: Snow, Water, Ice and Permafrost in the Arctic (SWIPA).
Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway, pp. 445-
483.
Høye, T.T., E. Post, H. Meltofte, N.M. Schmidt, and M.C. Forchhammer, 2007:
Rapid advancement of spring in the High Arctic. Current Biology, 17(12), R449-
R451.
Hudson, J.M. and G.H. Henry, 2009: Increased plant biomass in a High Arctic heath
community from 1981 to 2008. Ecology, 90(10), 2657-2663.
Hueffler, K., A.J. Parkinson, R. Gerlach, and J. Berner, 2013: Zoonotic infections in
Alaska: disease prevalence, potential impact of climate change and
recommended actions for earlier disease detection, research, prevention and
control. International Journal of Circumpolar Health, 72, 19562, doi:10.3402/
ijch.v72i0.19562.
Hughes, K.A. and P. Convey, 2010: The protection of Antarctic terrestrial ecosystems
from inter- and intra-continental transfer of non-indigenous species by human
activities: a review of current systems and practices. Global Environmental
Change, 20(1), 96-112.
Hughes-Hanks, J.M., L.G. Rickard, C. Panuska, J.R. Saucier, T.M. O’Hara, L. Dehn, and
R.M. Rolland, 2005: Prevalence of Cryptosporidium spp. and Giardia spp. in five
marine mammal species. The Journal of Parasitology, 91(5), 1225-1228.
Hung, H., R. Kallenborn., K. Breivik, Y. Su, E. Brorström-Lundén, K. Olafsdottir, J.M.
Thorlacius, S. Leppänen, R. Bossi, H. Skov, S. Manø, G.W. Patton, G. Stern, E.
Sverko, and P. Fellin, 2010: Atmospheric monitoring of organic pollutants in the
Arctic under the Arctic Monitoring and Assessment Programme (AMAP): 1993-
2006. Science of the Total Environment, 408(15), 2854-2873.
Hunt Jr., G.L., K.O. Coyle, L.B. Eisner, E.V. Farley, R.A. Heintz, F. Mueter, J.M. Napp, J.E.
Overland, P.H. Ressler, S. Salo, and P.J. Stabeno, 2011: Climate impacts on eastern
Bering Sea foodwebs: a synthesis of new data and an assessment of the
Oscillating Control Hypothesis. International Council for the Exploration of the
Sea (ICES) Journal of Marine Science, 68(6), 1230-1243.
Hunt Jr., G.L., A.L. Blanchard, P. Boveng, P. Dalpadado, K.F. Drinkwater, L. Eisner, R.R.
Hopcroft, K.M. Kovacs, B.L. Norcross, P. Renaud, M. Reigstad, M. Renner, H.R.
Skjoldal, A. Whitehouse, and R.A. Woodgate, 2013: The Barents and Chukchi
Seas: comparison of two Arctic shelf ecosystems. Journal of Marine Systems,
109-110, 43-68.
Hunter, C.M., H. Caswell, M.C. Runge, E.V. Regehr, S.C. Amstrup, and I. Stirling, 2010:
Climate change threatens polar bear populations: a stochastic demographic
analysis. Ecology, 91(10), 2883-2897.
Huntington, H., L. Hamilton, C. Nicolson, R. Brunner, A. Lynch, A. Ogilvie, and A.
Voinov, 2007: Toward understanding the human dimensions of the rapidly
changing arctic system: insights and approaches from five HARC projects.
Regional Environmental Change, 7(4), 173-186.
Huntington, O.H. and A. Watson, 2012: Interdisciplinarity, native resilience, and how
the riddles can teach wildlife law in an era of rapid climate change. Wicazōa
Review, 27(2), 49-73.
Hurst, T.P., E.R. Fernandez, J.T. Mathis, J.A. Miller, C.M. Stinson, and E.F. Ahgeak, 2012:
Resiliency of juvenile walleye pollock to projected levels of ocean acidification.
Aquatic Biology, 17, 247-259.
Huse, G. and I. Ellingsen, 2008: Capelin migrations and climate change – a modelling
analysis. Climatic Change, 87(1-2), 177-197.
IAATO, 2012: IAATO Overview of Antarctic Tourism: 2011-12 Season and Preliminary
Estimates for 2012-13 Season. Antarctic Treaty Consultative Meeting XXXV,
Hobart, Australia, Information Paper 39, International Association of Antarctic
Tour Operators (IAATO), Newport, RI, USA, 19 pp.
Ignatowski, J. and J. Rosales, 2013: Identifying the exposure of two subsistence
villages in Alaska to climate change using traditional ecological knowledge.
Climatic Change, 121(2), 285-299.
Ims, R.A., N.G. Yoccoz, K.A. Bråthen, P. Fauchald, T. Tveraa, and V. Hausner, 2007: Can
reindeer overabundance cause a trophic cascade? Ecosystems, 10(4), 607-
622.
Ims, R.A., N.G. Yoccoz, and S.T. Killengreen, 2011: Determinants of lemming
outbreaks. Proceedings of the National Academy of Sciences of the United
States of America, 108(5), 1970-1974.
Ingram, K.T., M.C. Roncoli, and P.H. Kirshen, 2002: Opportunities and constraints for
farmers of west Africa to use seasonal precipitation forecasts with Burkina Faso
as a case study. Agricultural Systems, 74(3), 331-349.
IPCC, 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working
Group I to the Fourth Assessment Report of the Intergovernmental Panel on
Climate Change [Solomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B.
Averyt, M. Tignor, and H.L. Miller (eds.)]. Cambridge University Press, Cambridge,
UK and New York, NY, USA, 996 pp.
IPCC, 2012: Managing the Risks of Extreme Events and Disasters to Advance Climate
Change Adaptation. A Special Report of Working Groups I and II of the
Intergovernmental Panel on Climate Change [Field, C.B., V. Barros, T.F. Stocker,
D. Qin, D.J. Dokken, K.L. Ebi, M.D. Mastrandrea, K.J. Mach, G.-K. Plattner, S.K.
Allen, M. Tignor, and P.M. Midgley (eds.)]. Cambridge University Press, Cambridge,
UK and New York, NY, USA, 582 pp.
Irons, D.B., T. Anker-Nilssen, A.J. Gaston, G.V. Byrd, K. Falk, G. Gilchrist, M. Hario, M.
Hjernquist, Y.V. Krasnov, A. Mosbech, B. Olsen, A. Petersen, J.B. Reid, G.J. Robertson,
H. Strom, and K.D. Wohl, 2008: Fluctuations in circumpolar seabird populations
linked to climate oscillations. Global Change Biology, 14(7), 1455-1463.
Iverson, S.J., I. Stirling, and S.L.C. Lang, 2006: Spatial and temporal variation in the
diets of polar bears across the Canadian Arctic: indicators of changes in prey
populations and environment. In: Top Predators in Marine Ecosystems [Boyd,
I.L., S. Wanless, and C.J. Camphuysen (eds.)]. Cambridge University Press,
Cambridge, UK, pp. 98-117.
Jansson, M., A. Jonsson, A. Andersson, and J. Karlsson, 2010: Biomass and structure
of planktonic communities along an air temperature gradient in subarctic
Sweden. Freshwater Biology, 55(3), 691-700.
Jarvis, T., N. Kelly, S. Kawaguchi, E. van Wijk, and S. Nicol, 2010: Acoustic characterisation
of the broad-scale distribution and abundance of Antarctic krill (Euphausia
superba) off East Antarctica (30-80°E) in January-March 2006. Deep-Sea
Research Part II: Topical Studies in Oceanography, 57(9-10), 916-933.
Jenouvrier, S., C. Barbraud, B. Cazelles, and H. Weimerskirch, 2005a: Modelling
population dynamics of seabirds: importance of the effects of climate fluctuations
on breeding proportions. Oikos, 108(3), 511-522.
Jenouvrier, S., H. Weimerskirch, C. Barbraud, Y.H. Park, and B. Cazelles, 2005b:
Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to
climate. Proceedings of the Royal Society B, 272(1566), 887-895.
Jenouvrier, S., H. Caswell, C. Barbraud, M. Holland, J. Stroeve, and H. Weimerskirch,
2009: Demographic models and IPCC climate projections predict the decline
of an emperor penguin population. Proceedings of the National Academy of
Sciences of the United States of America, 106(6), 1844-1847.
Jenouvrier, S., M. Holland, J. Stroeve, C. Barbraud, H. Weimerskirch, M. Serreze, and
H. Caswell, 2012: Effects of climate change on an emperor penguin population:
analysis of coupled demographic and climate models. Global Change Biology,
18(9), 2756-2770.
Jepsen, J.U., L. Kapari, S.B. Hagen, T. Schott, O.P.L. Vindstad, A.C. Nilssen, and R.A.
Ims, 2011: Rapid northwards expansion of a forest insect pest attributed to
spring phenology matching with sub-Arctic birch. Global Change Biology,
17(6), 2071-2083.
Jepsen, J.U., S.B. Hagen, R.A. Ims, and N.G. Yoccoz, 2008: Climate change and
outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in
subarctic birch forest: evidence of a recent outbreak range expansion. Journal
of Animal Ecology, 77(2), 257-264.
Jernsletten, J.-L.L. and K. Klokov, 2002: Sustainable Reindeer Husbandry. Arctic
Council and Centre for Saami Studies of the University of Tromsø, Tromsø,
Norway, 157 pp.
Ji, R., C.J. Ashjian, R.G. Campbell, C. Chen, G. Gao, C.S. Davis, G.W. Cowles, and R.C.
Beardsley, 2012: Life history and biogeography of Calanus copepods in the
Arctic Ocean: an individual-based modeling study. Progress in Oceanography,
96(1), 40-56.
Johansson, C., V.A. Pohjola, C. Jonasson, and T.V. Callaghan, 2011: Multi-decadal
changes in snow characteristics in sub-Arctic Sweden. AMBIO: A Journal of the
Human Environment, 40(6), 566-574.
Joly, K., D.R. Klein, D.L. Verbyla, T.S. Rupp, and F.S. Chapin, 2011: Linkages between
large-scale climate patterns and the dynamics of Arctic caribou populations.
Ecography, 34(2), 345-352.
Juday, G.P., 2009: Boreal forests and climate change. In: Oxford Companion to Global
Change [Goudie, A. and D. Cuff (eds.)]. Oxford University Press, Oxford, UK, pp.
75-84.
Kahru, M., V. Brotas, M. Manzano-Sarabia, and B.G. Mitchell, 2011: Are phytoplankton
blooms occurring earlier in the Arctic? Global Change Biology, 17(4), 1733-1739.
Kaplan, J.O. and M. New, 2006: Arctic climate change with a 2°C global warming: timing,
climate patterns and vegetation change. Climatic Change, 79(3-4), 213-241.

1604
Chapter 28 Polar Regions
28
Karnovsky, N., A. Harding, W. Walkusz, S. Kwasniewski, I. Goszczko, J. Wiktor, H.
Routti, A. Bailey, L. McFadden, Z. Brown, G. Beaugrand, and D. Gremillet, 2010:
Foraging distributions of little auks Alle alle across the Greenland Sea:
implications of present and future Arctic climate change. Marine Ecology
Progress Series, 415, 283-293.
Kausrud, K.L., A. Mysterud, H. Steen, J.O. Vik, E. Østbye, B. Cazelles, E. Framstad, A.M,
Eikeset, I. Mysterud, T. Solhøy, and N.C. Stenseth, 2008: Linking climate change
to lemming cycles. Nature, 456(7218), 93-97.
Kawaguchi, S., S. Nicol, and A.J. Press, 2009: Direct effects of climate change on the
Antarctic krill fishery. Fisheries Management and Ecology, 16(5), 424-427.
Kawaguchi, S., H. Kurihara, R. King, L. Hale, T. Berli, J.P. Robinson, A. Ishida, M. Wakita,
P. Virtue, S. Nicol, and A. Ishimatsu, 2011: Will krill fare well under Southern
Ocean acidification? Biology Letters, 7(2), 288-291.
Kawaguchi, S., A. Ishida, R. King, B. Raymond, N. Waller, A. Constable, S. Nicol, M.
Wakita, and A. Ishimatsu, 2013: Risk maps for Antarctic krill under projected
Southern Ocean acidification. Nature Climate Change, 3, 843-847.
Keskitalo, E., 2008: Vulnerability and adaptive capacity in forestry in northern Europe:
a Swedish case study. Climatic Change, 87(1), 219-234.
Keskitalo, E., 2009: Governance in vulnerability assessment: the role of globalising
decision-making networks in determining local vulnerability and adaptive capacity.
Mitigation and Adaptation Strategies for Global Change, 14(2), 185-201.
Kharuk, V.I., K.J. Ranson, S.T. Im, and M.M. Naurzbaev, 2006: Forest-tundra larch
forests and climatic trends. Russian Journal of Ecology, 37(5), 291-298.
Khon, V., I. Mokhov, M. Latif, V. Semenov, and W. Park, 2010: Perspectives of Northern
Sea Route and Northwest Passage in the twenty-first century. Climatic Change,
100(3), 757-768.
Kilpeläinen, A., H. Gregow, H. Strandman, S. Kellomäki, A. Venäläinen, and H. Peltola,
2010: Impacts of climate change on the risk of snow-induced forest damage
in Finland. Climatic Change, 99(1-2), 193-209.
Kitti, H., B.C. Forbes, and J. Oksanen, 2009: Long- and short-term effects of reindeer
grazing on tundra wetland vegetation. Polar Biology, 32(2), 253-261.
Kokelj, S.V., B. Zajdlik, and M.S. Thompson, 2009: The impacts of thawing permafrost
on the chemistry of lakes across the subarctic boreal-tundra transition, Mackenzie
Delta region, Canada. Permafrost and Periglacial Processes, 20(2), 185-199.
Kotwicki, S. and R.R. Lauth, 2013: Detecting temporal trends and environmentally-
driven changes in the spatial distribution of groundfishes and crabs on the eastern
Bering Sea shelf. Deep-Sea Research Part II: Topical Studies in Oceanography,
94, 155-175.
Kovacs, K.M. and C. Lydersen, 2008: Climate change impacts on seals and whales in the
North Atlantic Arctic and adjacent shelf seas. Science Progress, 91(Pt 2), 117-150.
Kovacs, K.M., C. Lydersen, J.E. Overland, and S.E. Moore, 2010: Impacts of changing sea-
ice conditions on Arctic marine mammals. Marine Biodiversity, 41(1), 181-194.
Krebs, C.J., 2011: Of lemmings and snowshoe hares: the ecology of northern Canada.
Proceedings of the Royal Society B, 278(1705), 481-489.
Kristiansen, T., K.F. Drinkwater, R.G. Lough, and S. Sundby, 2011: Recruitment
variability in North Atlantic cod and match-mismatch dynamics. PLoS ONE, 6(3),
e17456, doi:10.1371/journal.pone.0017456.
Krupnik, I. and D. Jolly (eds.), 2002: The Earth Is Faster Now: Indigenous Observations
of Arctic Environmental Change. Frontiers in Polar Social Science, Arctic
Research Consortium of the United States, Fairbanks, AK, USA, 383 pp.
Krupnik, I. and G.C. Ray, 2007: Pacific walruses, indigenous hunters, and climate
change: bridging scientific and indigenous knowledge. Deep-Sea Research Part
II: Topical Studies in Oceanography, 54(23-26), 2946-2957.
Krupnik, I., C. Aporta, S. Gearheard, G.J. Laidler, and L. Kielsen Holm (eds.), 2010:
SIKU: Knowing Our Ice –Documenting Inuit Sea Ice Knowledge and Use.
Springer, New York, NY, USA, 501 pp.
Krupnik, I., I. Allison, R. Bell, R. Cutler, D. Hik, J. López-Martínez, V. Rachold, E.
Sarukhanian, and C. Summerhayes (eds.), 2011: Understanding Earth’s Polar
Challenges: International Polar Year 2007-2008 – Summary by the IPY Joint
Committee. University of the Arctic Publications Series No. 4, International
Council for Science (ICSU)/World Meteorological Organization (WMO) Joint
Committee for International Polar Year 2007-2008, CCI Press, Edmonton, AB,
Canada and University of the Arctic, Rovaniemi, Finland, 695 pp.
Kullman, L. and L. Öberg, 2009: Post-Little Ice Age tree line rise and climate warming
in the Swedish Scandes: a landscape ecological perspective. Journal of Ecology,
97(3), 415-429.
Kumpula, T., A. Pajunen, E. Kaarlejärvi, B.C. Forbes, and F. Stammler, 2011: Land use
and land cover change in Arctic Russia: ecological and social implications of
industrial development. Global Environmental Change, 21(2), 550-562.
Kumpula, T., B.C. Forbes, F. Stammler, and N. Meschtyb, 2012: Dynamics of a coupled
system: multi-resolution remote sensing in assessing social-ecological
responses during 25 years of gas field development in Arctic Russia. Remote
Sensing, 4(4), 1046-1068.
Lack,D.A. and J.J. Corbett, 2012: Black carbon from ships: a review of the effects of
ship speed, fuel quality and exhaust gas scrubbing. Atmospheric Chemistry and
Physics, 12, 3985-4000.
Laidler, G.J., T. Hirose, M. Kapfer, T. Ikummaq, E. Joamie, and P. Elee, 2011: Evaluating
the Floe Edge Service: how well can SAR imagery address Inuit community
concerns around sea ice change and travel safety? Canadian Geographer / Le
Géographe Canadien, 55(1), 91-107.
Laidre, K.L., I. Stirling, L.F. Lowry, Ø. Wiig, M.P. Heide-Jørgensen, and S.H. Ferguson,
2008: Quantifying the sensitivity of arctic marine mammals to climate-induced
habitat change. Ecological Applications, 18(Suppl.), S97-S125.
Landerer, F.W., J.O. Dickey, and A. Güntner, 2010: Terrestrial water budget of the
Eurasian pan-Arctic from GRACE satellite measurements during 2003-2009.
Journal of Geophysical Research: Atmospheres, 115(D23), D23115, doi:10.1029/
2010JD014584.
Lantz, T.C. and S.V. Kokelj, 2008: Increasing rates of retrogressive thaw slump activity
in the Mackenzie Delta region, N.W.T., Canada. Geophysical Research Letters,
35(6), L06502, doi:10.1029/2007GL032433.
Larsen, J.N. and L. Huskey, 2010: Material wellbeing in the Arctic. In: Arctic Social
Indicators: A Follow-Up to the Arctic Human Development Report [Larsen, J.N.,
P. Schweitzer, and G. Fondahl (eds.)]. TemaNord 2010:519, Nordic Council of
Ministers, Copenhagen, Denmark, pp. 47-66.
Larsen, J.N., P. Schweitzer, and G. Fondahl (eds.), 2010: Arctic Social Indicators: A
Follow-Up to the Arctic Human Development Report. TemaNord 2010:519,
Nordic Council of Ministers, Copenhagen, Denmark,160 pp.
Laurion, I., W.F. Vincent, S. MacIntyre, L. Retamal, C. Dupont, P. Francus, and R. Pienitz,
2010: Variability in greenhouse gas emissions from permafrost thaw ponds.
Limonology and Oceanography, 55(1), 115-133.
Lee, S., M. Jin, and T. Whitledge, 2010: Comparison of bottom sea-ice algal
characteristics from coastal and offshore regions in the Arctic Ocean. Polar
Biology, 33(10), 1331-1337.
Lesack, L.F.W. and P. Marsh, 2007: Lengthening plus shortening of river-to-lake
connection times in the Mackenzie River Delta respectively via two global
change mechanisms along the arctic coast. Geophysical Research Letters,
34(23), L23404, doi:10.1029/2007GL031656.
Lesack, L.F.W. and P. Marsh, 2010: River-to-lake connectivities, water renewal, and
aquatic habitat diversity in the Mackenzie River Delta. Water Resources
Research, 46(12), W12504, doi:10.1029/2010WR009607.
Levintova, M, W.I. Zapol, and N. Engmann (eds.), 2010: Behavioral and mental health
research in the Arctic: a strategy setting meeting. International Journal of
Circumpolar Health, 5(Suppl.), 64 pp.
Lewis, T. and S.F. Lamoureux, 2010: Twenty-first century discharge and sediment yield
predictions in a small high Arctic watershed. Global and Planetary Change,
71(1-2), 27-41.
Li, W.K.W., F.A. McLaughlin, C. Lovejoy, and E.C. Carmack, 2009: Smallest algae thrive
as Arctic Ocean freshens. Science, 326(5952), 539, doi:10.1126/science.1179798.
Lima, M. and S.A. Estay, 2013: Warming effects in the western Antarctic Peninsula
ecosystem: the role of population dynamic models for explaining and predicting
penguin trends. Population Ecology, 55(4), 557-565.
Lind, S. and R.B. Ingvaldsen, 2012: Variability and impacts of Atlantic water entering
the Barents Sea from the north. Deep-Sea Research Part I: Oceanographic
Research Papers, 62, 70-88.
Lindgren, E.andR.Gustafson, 2001: Tick-borne encephalitis in Sweden and climate
change. The Lancet, 358(9275), 16-18.
Lindholt, L., 2006: Arctic natural resources in a global perspective. In: The Economy
of the North [Glomsrød, S. and I. Aslaksen (eds.)]. Statistics Norway, Oslo,
Norway, pp. 27-39.
Lindholdt, L. and S. Glomsrød, 2012: The Arctic: no big bonanza for the global
petroleum industry. Energy Economics, 34(5), 1465-1474.
Livingston, P., K. Aydin, J.L. Boldt, A.B. Hollowed, and J.M. Napp, 2011: Alaska marine
fisheries management: advancements and linkages to ecosystem research. In:
Ecosystem Based Management: An Evolving Perspective [Belgrano, A. and C.
Fowler (eds.)]. Cambridge University Press, Cambridge, UK, pp. 113-152.
Lloyd, A.H., A.G. Bunn, and L. Berner, 2011: A latitudinal gradient in tree growth
response to climate warming in the Siberian taiga. Global Change Biology,
17(5), 1935-1945.

1605
Polar Regions Chapter 28
28
Long, W.C., K.M. Swiney, C. Harris, H.N. Page, and R.J. Foy, 2013: Effects of ocean
acidification on juvenile red king crab (Paralithodes camtschaticus) and Tanner
crab (Chionoecetes bairdi) growth, condition, calcification, and survival. PLoS
ONE, 8(4), e60959, doi:10.1371/journal.pone.0060959.
Lorenzen, E.D., D. Nogués-Bravo, L. Orlando, J. Weinstock, J. Binladen, K.A. Marske,
A. Ugan, M.K. Borregaard, M.T.P. Gilbert, R. Nielsen, S.Y.W. Ho, T. Goebel, K.E.
Graf, D. Byers, J.T. Stenderup, M. Rasmussen, P.F. Campos, J.A. Leonard, K.-P.
Koepfli, D. Froese, G. Zazula, T.W. Stafford, K. Aaris-Sørensen, P. Batra, A.M.
Haywood, J.S. Singarayer, P.J. Valdes, G. Boeskorov, J.A. Burns, S.P. Davydov, J.
Haile, D.L. Jenkins, P. Kosintsev, T. Kuznetsova, X. Lai, L.D. Martin, H.G. McDonald,
D. Mol, M. Meldgaard, K. Munch, E. Stephan, M. Sablin, R.S. Sommer, T. Sipko,
E. Scott, M.A. Suchard, A. Tikhonov, R. Willerslev, R.K. Wayne, A. Cooper, M.
Hofreiter, A. Sher, B. Shapiro, C. Rahbek, and E. Willerslev, 2011: Species-specific
responses of Late Quaternary megafauna to climate and humans. Nature,
479(7373), 359-364.
Lund, D.H., K. Sehested, T. Hellesen, and V. Nellemann, 2012: Climate change
adaptation in Denmark: enhancement through collaboration and meta-
governance? Local Environment, 17(6-7), 613-628.
Lynch, H.J., R. Naveen, P.N. Trathan, and W.F. Fagan, 2012: Spatially integrated
assessment reveals widespread changes in penguin populations on the
Antarctic Peninsula. Ecology, 93(6), 1367-1377.
Lynn, K., J. Daigle, J. Hoffman, F. Lake, N. Michelle, D. Ranco, C. Viles, G. Voggesser,
and P. Williams, 2013: The impacts of climate change on tribal traditional foods.
Climatic Change, 120(3), 545-556.
Lyons, W.B., J. Laybourn-Parry, K.A. Welch, and J.C. Priscu, 2006: Antarctic lake
systems and climate change. In: Trends in Antarctic Terrestrial and Limnetic
Ecosystems: Antarctica as a Global Indicator [Bergstrom, D.M., P. Convey, and
A.H.L. Huiskes (eds.)]. Springer, Dordrecht, Netherlands, pp. 273-295.
Ma, J., H. Hung, C. Tian, and R. Kallenborn, 2001:Revolatilization of persistent organic
pollutants in the Arctic induced by climate change. Nature Climate Change, 1,
255-260.
MacCracken, J.G., 2012: Pacific walrus and climate change: observations and
predictions. Ecology and Evolution, 2(8), 2072-2090.
MacDonald, G.M., K.V. Kremenetski, and D.W. Beilman, 2008: Climate change and
the northern Russian treeline zone. Philosophical Transactions of the Royal
Society B, 363(1501), 2285-2299.
Macias-Fauria, M., B.C. Forbes, P. Zetterberg, and T. Kumpula, 2012: Eurasian Arctic
greening reveals teleconnections and the potential for novel ecosystems.
Nature Climate Change, 2, 613-618.
Mack, M.C., M.S. Bret-Harte, T.N. Hollingsworth, R.R. Jandt, E.A.G. Schuur, G.R. Shaver,
and D.L. Verbyla, 2011: Carbon loss from an unprecedented Arctic tundra
wildfire. Nature, 475(7357), 489-492.
Mackey, A.P., A. Atkinson, S.L. Hill, P. Ward, N.J. Cunningham, N.M. Johnston, and E.J.
Murphy, 2012: Antarctic macrozooplankton of the southwest Atlantic sector
and Bellingshausen Sea: baseline historical distributions (Discovery Investigations,
1928-1935) related to temperature and food, with projections for subsequent
ocean warming. Deep-Sea Research Part II: Topical Studies in Oceanography,
59, 130-146.
Magga, O.H., S.D. Mathiesen, R.W. Corell, and A. Oskal, 2011: Reindeer Herding,
Traditional Knowledge and Adaptation to Climate Change and Loss of Grazing
Land. Part of the Ipy Ealát Consortium, the Ealát Project (Ipy # 399), Reindeer
Herders Vulnerability Network Study: Reindeer Pastoralism in a Changing
Climate, A Project led by Norway and the Association of World Reindeer Herders
(WRH) in Arctic Council, Sustainable Development Working Group (SDWG),
International Centre for Reindeer Husbandry, Kautokeino, Norway, 75 pp.
Mahoney, A., S. Gearheard, T. Oshima, and T. Qillaq, 2009: Sea ice thickness
measurements from a community-based observing network. Bulletin of the
American Meteorological Society, 90(3), 370-377.
Maldonado, J.K., C. Shearer, R. Bronen, K. Peterson, and H. Lazrus, 2013: The impact
of climate change on tribal communities in the US: displacement, relocation,
and human rights. Climatic Change, 120(3), 601-614.
Mamet, S.D. and G.P. Kershaw, 2012: Subarctic and alpine tree line dynamics during
the last 400 years in north-western and central Canada. Journal of Biogeography,
39(5), 855-868.
Marsh, P., M. Russell, S. Pohl, H. Haywood, and C. Onclin, 2009: Changes in thaw lake
drainage in the western Canadian Arctic from 1950 to 2000. Hydrological
Processes, 23(1), 145-158.
Massom, R.A. and S.E. Stammerjohn, 2010: Antarctic sea ice change and variability
– physical and ecological implications. Polar Science, 4(2), 149-186.
Mathiesen, S.D., B. Alfthan, R. Corell, R.B.M. Eira, I.M.G. Eira, A. Degteva, K.I. Johnsen,
A. Oskal, M. Roué, M.N. Sara, E.R. Skum, E.I. Tury, and J.M. Turi, 2013: Strategies
to enhance the resilience of Sámi reindeer husbandry to rapid changes in the
Arctic. In: Arctic Resilience Interim Report 2013. Stockholm Environment Institute
(SEI) and Stockholm Resilience Centre, Stockholm, Sweden, pp. 109-112.
Matrai, P.A., E. Olson, S. Suttles, V. Hill, L.A. Codispoti, B. Light, and M. Steele, 2013:
Synthesis of primary production in the Arctic Ocean: I. Surface waters, 1954-
2007. Progress in Oceanography, 110, 93-106.
Matthews, C., S. Luque, S. Petersen, R. Andrews, and S. Ferguson, 2011: Satellite
tracking of a killer whale (Orcinus orca) in the eastern Canadian Arctic documents
ice avoidance and rapid, long-distance movement into the North Atlantic. Polar
Biology, 34(7), 1091-1096.
Maynard, N.G., 2006: Satellites, settlements, and human health. In: Remote Sensing
of Human Settlements [Ridd, M. and J.D. Hipple (eds.)]. Manual of Remote
Sensing, 3
rd
edn., Vol. 5, American Society of Photogrammetry and Remote
Sensing (ASPRS), Bethesda, MD, USA, pp. 379-399.
Maynard, N.G. and G.A. Conway, 2007: A view from above: use of satellite imagery
to enhance our understanding of potential impacts of climate change on human
health in the Arctic. Alaska Medicine, 49(2 Suppl.), 38-43.
Maynard, N.G., A. Oskal, J.M. Turi, S.D. Mathiesen, I.M.G. Eira, B. Yurchak, V. Etylin,
and J. Gebelein, 2011: Impacts of Arctic climate and land use changes on
reindeer pastoralism: indigenous knowledge and remote sensing. In: Eurasian
Arctic Land Cover and Land Use in a Changing Climate [Gutman, G. and A.
Reissell (eds.)]. Springer, Dordrecht, Netherlands, pp. 177-205.
McLaughlin, J.B., A. DePaola, C.A. Bopp, K.A. Martinek, N.P. Napolilli, C.G. Allison,
S.L. Murray, E.C. Thompson, M.M. Bird, and J.P. Middaugh, 2005: Outbreak of
Vibrio parahaemolyticus gastroenteritis associated with Alaskan oysters. New
England Journal of Medicine, 353(14), 1463-1470.
McLeod, D., G. Hallegraeff, G. Hosie, and A. Richardson, 2012: Climate-driven range
expansion of the red-tide dinoflagellate Noctiluca scintillans into the Southern
Ocean. Journal of Plankton Research 34(4), 332-337.
McNamara, J.P. and D.L. Kane, 2009: The impact of a shrinking cryosphere on the
form of arctic alluvial channels. Hydrological Processes, 23(1), 159-168.
McNeeley, S.M., 2012: Examining barriers and opportunities for sustainable
adaptation to climate change in Interior Alaska. Climatic Change, 111(3-4),
835-857.
Mehlum, F. 2012: Effects of sea ice on breeding numbers and clutch size of a High
Arctic population of the common eider Somateria mollissima. Polar Science,
6(1), 143-153.
Melbourne-Thomas, J., A. Constable, S. Wotherspoon, and B. Raymond, 2013: Testing
paradigms of ecosystem change under climate warming in Antarctica. PLoS
ONE, 8(2), e55093, doi:10.1371/journal.pone.0055093.
Melles, M., J. Brigham-Grette, O.Y. Glushkova, P.S. Minyuk, N.R. Nowaczyk, and
H.-W. Hubberten, 2007: Sedimentary geochemistry of core PG1351 from Lake
El’gygytgyn – a sensitive record of climate variability in the East Siberian Arctic
during the past three glacial-interglacial cycles. Journal of Paleolimnology,
37(1), 89-104.
Merino, G., M. Barange, J.L. Blanchard, J. Harle, R. Holmes, I. Allen, E.H. Allison, M.C.
Badjeck, N.K. Dulvy, J. Holt, S. Jennings, C. Mullon, and L.D. Rodwell, 2012: Can
marine fisheries and aquaculture meet fish demand from a growing human
population in a changing climate? Global Environmental Change, 22(4), 795-806.
Meschtyb, N., B. Forbes, and P. Kankaanpää, 2005: Social impact assessment along
Russia’s Northern Sea Route: petroleum transport and the Arctic Operational
Platform (ARCOP). Arctic, 58(3), 322-327.
Mesquita, P.S., F.J. Wrona, and T.D. Prowse, 2010: Effects of retrogressive permafrost
thaw slumping on sediment chemistry and submerged macrophytes in Arctic
tundra lakes. Freshwater Biology, 55(11), 2347-2358.
Metje, M. and P. Frenzel, 2007: Methanogenesis and methanogenic pathways in a
peat from subarctic permafrost. Environmental Microbiology, 9(4), 954-964.
Miller, F.L. and S.J. Barry, 2009: Long-term control of Peary caribou numbers by
unpredictable, exceptionally severe snow or ice conditions in a non-equilibrium
grazing system. Arctic, 62(2), 175-189.
Miller, W., S.C. Schuster, A.J. Welch, A. Ratan, O.C. Bedoya-Reina, F. Zhao, H.L. Kim,
R.C. Burhans, D.I. Drautz, N.E. Wittekindt, L.P. Tomsho, E. Ibarra-Laclette, L.
Herrera-Estrella, E. Peacock, S. Farley, G.K. Sage, K. Rode, M. Obbard, R. Montiel,
L. Bachmann, Ó. Ingólfsson, J. Aars, T. Mailund, Ø. Wiig, S.L. Talbot, and C.
Lindqvist, 2012: Polar and brown bear genomes reveal ancient admixture and
demographic footprints of past climate change. Proceedings of the National
Academy of Sciences of the United States of America, 109(36), E2382-E2390

1606
Chapter 28 Polar Regions
28
Milliman, J.D., K.L. Farnsworth, P.D. Jones, K.H. Xu, and L.C. Smith, 2008: Climatic
and anthropogenic factors affecting river discharge to the global ocean, 1951-
2000. Global and Planetary Change, 62(3-4), 187-194.
Milner, A.M., L.E. Brown, and D.M. Hannah, 2009: Hydroecological response of river
systems to shrinking glaciers. Hydrological Processes, 23(1), 62-77.
Moe, B., L. Stempniewicz, D. Jakubas, F. Angelier, O. Chastel, F. Dinessen, G.W.
Gabrielsen, F. Hanssen, N.J. Karnovsky, B. Ronning, J. Welcker, K. Wojczulanis-
Jakubas, and C. Bech, 2009: Climate change and phenological responses of
two seabird species breeding in the High-Arctic. Marine Ecology Progress Series,
393, 235-246.
Moen, J., K. Aune, L. Edenius, and A. Angerbjörn, 2004: Potential effects of climate
change on treeline position in the Swedish mountains. Ecology and Society,
9(1), www.ecologyandsociety.org/vol9/iss1/art16/.
Mokhov, I.I. and V.C. Khon, 2008: Assessment of the Northern Sea Route perspectives
under climate changes on the basis of simulations with the climate models
ensemble. In: Natural Processes in Polar Regions, Part 2 [Kotlijakov, V.M. (ed.)].
Vol. III of the Changes of Natural Environment and Climate: Natural and
Possible Consequent Human-induced Catastrophes Series, The Institute of
Geography (IG), Russian Academy of Sciences (RAS), IG RAS, Moscow, Russia,
pp. 20-27.
Molenaar, E.J., 2009: Climate change and Arctic fisheries. In: Climate Governance in
the Arctic [Koivurova, T., E.C.H. Keskitalo, and N. Bankes (eds.)]. Springer,
Dordrecht, Netherlands, pp. 145-169.
Moline, M.A., N.J. Karnovsky, Z. Brown, G.J. Divoky, T.K. Frazer, C.A. Jacoby, J.J. Torrese,
and W.R. Fraser, 2008: High latitude changes in ice dynamics and their impact
on polar marine ecosystems. Annals of the New York Academy of Sciences,
1134(1), 267-319.
Molnar, P.K., A.E. Derocher, T. Klanjscek, and M.A. Lewis, 2011: Predicting climate
change impacts on polar bear litter size. Nature Communications, 2, 186,
doi:10.1038/ncomms1183.
Monnett, C. and J. Gleason, 2006: Observations of mortality associated with
extended open-water swimming by polar bears in the Alaskan Beaufort Sea.
Polar Biology, 29(8), 681-687.
Montes-Hugo, M., S.C. Doney, H.W. Ducklow, W. Fraser, D. Martinson, S.E. Stammerjohn,
and O. Schofield, 2009: Recent changes in phytoplankton communities associated
with rapid regional climate change along the Western Antarctic Peninsula.
Science, 323(5920), 1470-1473.
Moore, R.D., S.W. Fleming, B. Menounos, R. Wheate, A. Fountain, K. Stahl, K. Holm,
and M. Jakob, 2009: Glacier change in western North America: influences on
hydrology, geomorphic hazards and water quality. Hydrological Processes,
23(25), 42-61.
Moore, S.E., 2008: Marine mammals as ecosystem sentinels. Journal of Mammology,
89(3), 534-540.
Moore, S.E. and H.P. Huntington, 2008: Arctic marine mammals and climate change:
impacts and resilience. Ecological Applications: A Publication of the Ecological
Society of America, 18(2 Suppl.), s157-s165.
Morán, X.A.G., Á. López-Urrutia, A. Calvo-Díaz, and W.K.W. Li, 2010: Increasing
importance of small phytoplankton in a warmer ocean. Global Change Biology,
16(3), 1137-1144.
Moy, A.D., W.R. Howard, S.G. Bray, and T.W. Trull, 2009: Reduced calcification in
modern Southern Ocean planktonic foraminifera. Nature Geoscience, 2(4), 276-
280.
Mueter, F.J. and M.A. Litzow, 2008: Sea ice retreat alters the biogeography of the
Bering Sea continental shelf. Ecological Applications, 18(2), 309-320.
Mueter, F.J., J.L. Boldt, B.A. Megrey, and R.M. Peterman, 2007: Recruitment and
survival of northeast Pacific Ocean fish stocks: temporal trends, covariation,
and regime shifts. Canadian Journal of Fisheries and Aquatic Sciences, 64(6),
911-927.
Mueter, F.J., N.A. Bond, J.N. Ianelli, and A.B. Hollowed, 2011: Expected declines in
recruitment of walleye pollock (Theragra chacogramma) in the eastern Bering
Sea under future climate change. International Council for the Exploration of
the Sea (ICES) Journal of Marine Science, 68(6), 1284-1296.
Mundy, P.R. and D.F.Evenson, 2011: Environmental controls of phenology of high-
latitude Chinook salmon populations of the Yukon River, North America, with
application to fishery management. International Council for the Exploration
of the Sea (ICES) Journal of Marine Science, 68(6), 1155-1164.
Murphy, E., J. Watkins, P. Trathan, K. Reid, M. Meredith, S. Thorpe, N. Johnston, A.
Clarke, G. Tarling, M. Collins, J. Forcada, R. Shreeve, A. Atkinson, R. Korb, M.
Whitehouse, P. Ward, P. Rodhouse, P. Enderlein, A. Hirst, A. Martin, S. Hill, I.
Staniland, D. Pond, D. Briggs, N. Cunningham, and A. Fleming, 2007: Spatial
and temporal operation of the Scotia Sea ecosystem: a review of large-scale
links in a krill centred food web. Philosophical Transactions of the Royal Society
B, 362(1477), 113-148.
Murphy, E.J., R.D. Cavanagh, E.E. Hofmann, S.L. Hill, A.J. Constable, D.P. Costa, M.H.
Pinkerton, N.M. Johnston, P.N. Trathan, J.M. Klinck, D.A. Wolf-Gladrow, K.L. Daly,
O. Maury, and S.C. Doney, 2012a: Developing integrated models of Southern
Ocean food webs: including ecological complexity, accounting for uncertainty
and the importance of scale. Progress in Oceanography, 102, 74-92.
Murphy, E.J., J.L. Watkins, P.N. Trathan, K. Reid, M.P. Meredith, S.L. Hill, S.E. Thorpe, N.M.
Johnston, A. Clarke, G.A. Tarling, M.A. Collins, J. Forcada, A. Atkinson, P. Ward, I.J.
Staniland, D.W. Pond, R.A. Cavanagh, R.S. Shreeve, R.E. Korb, M.J. Whitehouse,
P.G. Rodhouse, P. Enderlein, A.G. Hirst, A.R. Martin, D.R. Briggs, N.J. Cunningham,
and A.H. Fleming, 2012b: Spatial and temporal operation of Scotia Sea ecosystem.
In: Antarctic Ecosystems [Rogers, A., N. Johnston, E. Murphy, and A. Clarke (eds.)].
Wiley-Blackwell, Chichester, UK, and Hoboken, NJ, USA, pp. 160-212.
Murphy, E.J., E.E. Hofmann, J.L. Watkins, N.M. Johnston, A. Piñones, T. Ballerini, S.L.
Hill, P.N. Trathan, G.A. Tarling, R.A. Cavanagh, E.F. Young, S.E. Thorpe, and P.
Fretwell, 2013: Comparison of the structure and function of Southern Ocean
regional ecosystems: the Antarctic Peninsula and South Georgia. Journal of
Marine Systems, 109-110, 22-42.
Myers-Smith, I.H., B.C. Forbes, M. Wilmking, M. Hallinger, T. Lantz, D. Blok, K.D. Tape,
M. MacIas-Fauria, U. Sass-Klaassen, E. Lévesque, S. Boudreau, P. Ropars, L.
Hermanutz, A. Trant, L.S. Collier, S. Weijers, J. Rozema, S.A. Rayback, N.M.
Schmidt, G. Schaepman-Strub, S. Wipf, C. Rixen, C.B. Ménard, S. Venn, S. Goetz,
L. Andreu-Hayles, S. Elmendorf, V. Ravolainen, J. Welker, P. Grogan, H.E. Epstein,
and D.S. Hik, 2011a: Shrub expansion in tundra ecosystems: dynamics, impacts
and research priorities. Environmental Research Letters, 6(4), 045509,
doi:10.1088/1748-9326/6/4/045509.
Myers-Smith, I.H., D.S. Hik, C. Kennedy, D. Cooley, J.F. Johnstone, A.J. Kenney, and
C.J. Krebs, 2011b: Expansion of canopy-forming willows over the twentieth
century on Herschel Island, Yukon Territory, Canada. AMBIO: A Journal of the
Human Environment, 40(6), 610-623.
Nakashima, D.J., K.G. McLean, H. Thulstrup, R.C. Ameyali, and J. Rubis, 2012: Indigenous
Knowledge, Marginalized Peoples and Climate Change: Foundations for
Assessment and Adaptation. Technical Report prepared for Working Group II
of the Intergovernmental Panel on Climate Change, United Nations Educational,
Scientific, and Cultural Organization (UNESCO), Paris, France, and United
Nations University (UNU), Darwin, Australia, 82 pp.
Nayha, S., 2005: Environmental temperature and mortality. International Journal of
Circumpolar Health, 64(5), 451-458.
Nicol, S. and A. Brierley, 2010: Through a glass less darkly – new approaches for
studying the distribution, abundance and biology of euphausiids. Deep-Sea
Research Part II: Topical Studies in Oceanography, 57(7-8), 496-507.
Nicol, S., T. Pauly, N.L. Bindoff, S. Wright, D. Thiele, G.W. Hosie, P.G. Strutton, and E.
Woehler, 2000a: Ocean circulation off east Antarctica affects ecosystem structure
and sea-ice extent. Nature, 406(6795), 504-507.
Nicol, S., A.J. Constable, and T. Pauly, 2000b: Estimates of circumpolar abundance of
Antarctic krill based on recent acoustic density measurements. Commission for
the Conservation of Antarctic Marine Living Resources Science, 7, 87-99.
Nicol, S., A. Worby, and R. Leaper, 2008: Changes in the Antarctic sea ice ecosystem:
potential effects on krill and baleen whales. Marine and Freshwater Research,
59(5), 361-382.
Nicol, S., J. Foster, and S. Kawaguchi, 2012: The fishery for Antarctic krill – recent
developments. Fish and Fisheries, 13(1), 30-40.
Nikanorov, A., V. Bryzgalo, and G. Chernogaeva, 2007: Anthropogenically modified
natural background and its formation in the Russian freshwater ecosystems.
Russian Meteorology and Hydrology, 32(11), 698-710.
NorACIA, 2010: Klimaendringer i Norsk Arktis – Konsekvenser for Livet i Nord. Norsk
Polarinstitutt (Norwegian Polar Institute) Report 136, Norwegian Arctic Climate
Impact Assessment (NorACIA), NorACIA Sekretariat, Norsk Polarinstitutt,
Tromsø, Norway, 131 pp. (in Norwegian).
NRTEE, 2009: True North: Adapting Infrastructure to Climate Change in Northern
Canada. National Round Table on the Environment and the Economy (NRTEE),
Ottawa, ON, Canada, 146 pp.
Nuttall, M., F. Berkes, B. Forbes, G. Kofinas, T. Vlassova, and G. Wenzel, 2005: Hunting,
herding, fishing and gathering: indigenous peoples and renewable resource
use in the Arctic. In: Arctic Climate Impact Assessment. Cambridge University
Press, New York, NY, USA, 649-690.

1607
Polar Regions Chapter 28
28
Ogden, N.H., C. Bouchard, K. Kurtenbach, G. Margos, L.R. Lindsay, L. Trudel, S. Nguon,
and F. Milord, 2010: Active and passive surveillance and phylogenetic analysis
of Borrelia burgdorferi elucidate the process of lyme disease risk emergence in
Canada. Environmental Health Perspectives, 118(7), 909-914.
Olech, M. and K.J. Chwedorzewska, 2011: Short note: the first appearance and
establishment of an alien vascular plant in natural habitats on the forefield of
a retreating glacier in Antarctica. Antarctic Science, 23(02), 153-154.
Olli, K., P. Wassmann, M. Reigstad, T.N. Ratkova, E. Arashkevich, A. Pasternak, P.A.
Matrai, J. Knulst, L. Tranvik, R. Klais, and A. Jacobsen, 2007: The fate of production
in the central Arctic Ocean – top-down regulation by zooplankton expatriates?
Progress in Oceanography, 72(1), 84-113.
Olofsson, A., O. Danell, P. Forslund, and B. Åhman, 2011: Monitoring changes in lichen
resources for range management purposes in reindeer husbandry. Ecological
Indicators, 11(5), 1149-1159.
Olofsson, J., L. Oksanen, T. Callaghan, P.E. Hulme, T. Oksanen, and O. Suominen, 2009:
Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change
Biology, 15(11), 2681-2693.
Olofsson, J., H. Tømmervik, and T.V. Callaghan, 2012: Vole and lemming activity
observed from space. Nature Climate Change, 2, 880-883.
Ormseth, O.A. and B.L. Norcross, 2009: Causes and consequences of life-history
variation in North American stocks of Pacific cod. International Council for the
Exploration of the Sea (ICES) Journal of Marine Science, 66(2), 349-357.
Orr, J.C., K. Caldeira, V. Fabry, J.-P. Gattuso, P. Haugan, P. Lethodey, S. Pantoja, H.-O.
Pörtner, U. Riebesell, T. Trull, M. Hood, E. Urban, and W. Broadgate, 2009:
Research priorities for understanding ocean acidification: summary from the
second symposium on the ocean in a high-CO
2
world. Oceanography, 22(4),
182-189.
Oskal, A., 2008: Old livelihoods in new weather: Arctic indigenous reindeer herders
face the challenges of climate change. Development Outreach, 10(1), 22-25.
Østreng, W., 2006: The International Northern Sea Route Programme (INSROP):
applicable lessons learned. Polar Record, 42(1), 71-81.
Outridge, P.M., H. Sanei, G.A. Stern, P.B. Hamilton, and F. Goodarzi, 2007: Evidence
for control of mercury accumulation rates in Canadian High Arctic lake
sediments by variations of aquatic primary productivity. Environmental Science
& Technology, 41(15), 5259-5265.
Overeem, I. and J.P.M. Syvitski, 2010: Shifting discharge peaks in Arctic rivers, 1977-
2007. Geografiska Annaler: Series A, Physical Geography, 92(2), 285-296.
Overland, J.E., J.A. Francis, E. Hanna, and M. Wang, 2012: The recent shift in early
summer Arctic atmospheric circulation. Geophysical Research Letters, 39,
L19804, doi:10.1029/2012GL053268.
Pagano, A.M., G.M. Durner, S.C. Amstrup, K.S. Simac, and G.S. York, 2012: Long-
distance swimming by polar bears (Ursusmaritimus) of the southern Beaufort
Sea during years of extensive open water. Canadian Journal of Zoology, 90(5),
663-676.
Parada, C., D.A. Armstrong, B. Ernst, S. Hinckley, and J.M. Orensanz, 2010: Spatial
dynamics of snow crab (Chionoecetes opilio) in the eastern Bering Sea – putting
together the pieces of the puzzle. Bulletin of Marine Science, 86(2), 413-437.
Parkinson, A.J., 2009: Sustainable development, climate change and human health
in the Arctic. In: Climate Change and Arctic Sustainable Development: Scientific,
Social, Cultural, and Educational Challenges. United Nations Educational,
Scientific, and Cultural Organization (UNESCO), Paris, France, pp. 156-163.
Parkinson, A.J. and B. Evengård, 2009: Climate change, its impact on human health
in the Arctic and the public health response to threats of emerging infectious
diseases. Global Health Action, 2, doi:10.3402/gha.v2i0.2075.
Parkinson, A.J., M.G. Bruce, and T. Zulz, 2008: International Circumpolar Surveillance,
an Arctic network for the surveillance of infectious diseases. Emerging Infectious
Diseases, 14(1), 18-24.
Parnikoza, I., P. Convey, I. Dykyy, V. Trokhymets, G. Milinevsky, O. Tyschenko, D.
Inozemtseva, and I. Kozeretska, 2009: Current status of the Antarctic herb
tundra formation in the Central Argentine Islands. Global Change Biology,
15(7), 1685-1693.
Paxian, A., V. Eyring, W. Beer, R. Sausen, and C. Wright, 2010: Present-day and future
global bottom-up ship emission inventories including polar routes. Environmental
Science & Technology, 44(4), 1333-1339.
Payette, S., 2007: Contrasted dynamics of northern Labrador tree lines caused by
climate change and migrational lag. Ecology, 88(3), 770-780.
Pearson, R.G., S.J. Phillips, M.M. Loranty, P.S.A. Beck, T. Damoulas, S.J. Knight, and
S.J. Goetz, 2013: Shifts in Arctic vegetation and associated feedbacks under
climate change. Nature Climate Change, 3, 673-677.
Peck, L.S., D.K.A. Barnes, A.J. Cook, A.H. Fleming, and A. Clarke, 2009: Negative
feedback in the cold: ice retreat produces new carbon sinks in Antarctica. Global
Change Biology, 16(9), 2614-2623.
Péron, C., M. Authier, C. Barbraud, K. Delord, D. Besson, and H. Weimerskirch, 2010:
Interdecadal changes in at-sea distribution and abundance of subantarctic
seabirds along a latitudinal gradient in the Southern Indian Ocean. Global
Change Biology, 16(7), 1895-1909.
Péron, C., H. Weimerskirch, and C.A. Bost, 2012: Projected poleward shift of king
penguins’ (Aptenodytes patagonicus) foraging range at the Crozet Islands,
southern Indian Ocean. Proceedings of the Royal Society B, 279(1738), 2515-
2523.
Perrette, M., A. Yool, G.D. Quartly, and E.E. Popova, 2011: Near-ubiquity of ice-edge
blooms in the Arctic. Biogeosciences, 8(2), 515-524.
Peters, G.P., T.B. Nilssen, L. Lindholdt, M.S. Eide, S. Glomsrød, L.I. Eide, and J.S.
Fuglestvedt, 2011: Future emissions from shipping and petroleum activities in
the Arctic. Atmospheric Chemistry and Physics, 11, 5305-5320.
Peterson, B.J., R.M. Holmes, J.W. McClelland, C.J. Vörösmarty, R.B. Lammers, A.I.
Shiklomanov, I.A. Shiklomanov, and S. Rahmstorf, 2002: Increasing river
discharge to the Arctic Ocean. Science, 298(5601), 2171-2173.
Pohl, S., P. Marsh, and B. Bonsal, 2007: Modeling the impact of climate change on
runoff and annual water balance of an Arctic headwater basin. Arctic, 60(2),
173-186.
Poloczanska, E.S., C.J. Brown, W.J. Sydeman, W. Kiessling, D. S. Schoeman, P.J. Moore,
K. Brander, J.F. Bruno, L.B. Buckley, M.T. Burrows, C.M. Duarte, B.S. Halpern, J.
Holding, C.V. Kappel, M.I. O’Connor, J.M. Pandolfi, C. Parmesan, F. Schwing, S.A.
Thompson, and A.J. Richardson, 2013: Global imprint of climate change on
marine life. Nature Climate Change, 3, 919-925.
Poppel, B. and J. Kruse, 2009: The importance of a mixed cash- and harvest herding
based economy to living the Arctic: an analysis on the Survey of Living Conditions
in the Arctic (SLiCA). In: Quality of Life in the New Milleium: Advances in the
Quality-of-Life Studies, Theory and Research [Møller, V. and D. Huschka (eds.)].
Social Indicators Research, Vol. 35, Springer, Dordrecht, Netherlands, pp. 27-42.
Portier, C.J., K. Thigpen Tart, S.R. Carter, C.H. Dilworth, A.E. Grambsch, J. Gohlke, J.
Hess, S.N. Howard, G. Luber, J.T. Lutz, T. Maslak, N. Prudent, M. Radtke, J.P.
Rosenthal, T. Rowles, P.A. Sandifer, J. Scheraga, P.J. Schramm, D. Strickman, J.M.
Trtanj, and P.-Y. Whung, 2010: A Human Health Perspective on Climate Change:
A Report Outlining the Research Needs on the Human Health Effects of Climate
Change. Prepared by the Interagency Working Group on Climate Change and
Health and published jointly by Environmental Health Perspectives and the
National Institute of Environmental Health Sciences (NIEHS), Research Triangle
Park, NC, USA, 71 pp.
Pörtner, H.-O. and M.A. Peck, 2010: Climate change effects on fishes and fisheries:
towards a cause-and-effect understanding. Journal of Fish Biology, 77(8), 1745-
1779.
Post, E. and M.C. Forchhammer, 2008: Climate change reduces reproductive success
of an Arctic herbivore through trophic mismatch. Philosophical Transactions of
the Royal Society B, 363(1501), 2369-2375.
Post, E., C. Pedersen, C.C. Wilmers, and M.C. Forchhammer, 2008: Warming, plant
phenology and the spatial dimension of trophic mismatch for large herbivores.
Proceedings of the Royal Society B, 275(1646), 2005-2013.
Post, E., J. Brodie, M. Hebblewhite, A.D. Anders, J.A.K. Maier, and C.C. Wilmers, 2009a:
Global population dynamics and hot spots of response to climate change.
BioScience, 59(6), 489-497.
Post, E., M.C. Forchhammer, M.S. Bret-Harte, T.V. Callaghan, T.R. Christensen, B.
Elberling, A.D. Fox, O. Gilg, D.S. Hik, T.T. Høye, R.A. Ims, E. Jeppesen, D.R. Klein,
J. Madsen, A.D. McGuire, S. Rysgaard, D.E. Schindler, I. Stirling, M.P. Tamstorf,
N.J.C. Tyler, R. van der Wal, J. Welker, P.A. Wookey, N.M. Schmidt, and P. Aastrup,
2009b: Ecological dynamics across the Arctic associated with recent climate
change. Science, 325(5946), 1355-1358.
Pouliot, J., P.E. Galand, C. Lovejoy, and W.F. Vincent, 2009: Vertical structure of
archaeal communities and the distribution of ammonia monooxygenase A gene
variants in two meromictic High Arctic lakes. Environmental Microbiology,
11(3), 687-699.
Prach, K., J. Košnar, J. Klimešová, and M. Hais, 2010: High Arctic vegetation after 70
years: a repeated analysis from Svalbard. Polar Biology, 33(5), 635-639.
Prowse, T.D. and K. Brown, 2010a: Appearing and disappearing lakes in the Arctic and
their impacts on biodiversity. In: Arctic Biodiversity Trends 2010: Selected Indicators
of Change [Kurvits, T., B. Alfthan, and E. Mork (eds.)]. Conservation of Arctic Flora
and Fauna (CAFF) International Secretariat, Akureyri, Iceland, pp. 68-70.

1608
Chapter 28 Polar Regions
28
Prowse, T.D. and K. Brown, 2010b: Hydro-ecological effects of changing Arctic river
and lake ice covers: a review. Hydrology Research, 41(6), 454-461.
Prowse, T.D., B.R. Bonsal, C.R. Duguay, D.O. Hessen, and V.S. Vuglinsky, 2007: River
and lake ice. In: Global Outlook for Ice and Snow. Division of Early Warning
and Assessment (DEWA), United Nations Environment Programme (UNEP) and
UNEP/Global Resource Information Database (GRID-Arendal), UNEP, Nairobi,
Kenya, pp. 201-214.
Prowse, T.D., C. Furgal, R. Chouinard, H. Melling, D. Milburn, and S.L. Smith, 2009:
Implications of climate change for economic development in northern Canada:
energy, resource, and transportation sectors. AMBIO: A Journal of the Human
Environment, 38(5), 272-281.
Prowse, T., K. Alfredsen, S. Beltaos, B. Bonsal, C. Duguay, A. Korhola, J. McNamara,
W. Vincent, V. Vuglinsky, G. Weyhenmeyer, K. Walter Anthony, B. Bowden, V.
Buzin, Y. Dibike, N. Gantner, L. Hinzman, L. Lia, T. Ouarda, R. Pientiz, J.D. Reist,
M. Stickler, J. Weckström, and F. Wrona, 2011. Chapter 6: Changing lake and
river ice regimes: trends, effects, and implications. In: Snow, Water, Ice, and
Permafrost in the Arctic (SWIPA): Climate Change and the Cryosphere. Scientific
Assessment of the Arctic Monitoring and Assessment Program (AMAP), Oslo,
Norway, pp. 6-1 to 6-52.
Quayle, W.C., P. Convey, L.S. Peck, C.J. Ellis-Evans, H.G. Butler, and H.J. Peat, 2013:
Ecological responses of maritime Antarctic lakes to regional climate change.
In:Antarctic Peninsula Climate Variability: Historical and Paleoenvironmental
Perspectives [Domack, E., A. Leventer, A. Burnett, R. Bindschadler, P. Convey,
and M. Kirby (eds.)]. Antarctic Research Series 79, American Geophysical
Union,Washington, DC, USA, pp. 159-170.
Quesada, A. and D. Velázquez, 2012: Global change effects on Antarctic freshwater
ecosystems: the case of maritime Antarctic lakes. In: Climatic Change and
Global Warming of Inland Waters: Impacts and Mitigation for Ecosystems and
Societies [Kumagai, M., C.R. Goldman, and R.D. Robarts (eds.)]. John Wiley &
Sons, Chichester, UK, pp. 367-382.
Quesada, A., W.F. Vincent, E. Kaup, J.E. Hobbie, I. Laurion, R. Pienitz, J. López-Martínez,
and J.J. Durán, 2006: Landscape control of high latitude lakes in a changing
climate. In: Trends in Antarctic Terrestrial and Limnetic Ecosystems. Antarctica
as a Global Indicator [Bergstrom, D.M., P. Convey, and A.H.L. Huiskes (eds.)].
Springer, Dordrecht, Netherlands, pp. 221-252.
Ragen, T.J., H.P. Huntington, and G.K. Hovelsrud, 2008: Conservation of Arctic marine
mammals faced with climate change. Ecological Applications, 18(2 Suppl.),
S166-S174.
Rawlins, M.A., H. Ye, D. Yang, A. Shiklomanov, and K.C. McDonald, 2009a: Divergence
in seasonal hydrology across northern Eurasia: emerging trends and water cycle
linkages. Journal of Geophysical Research: Atmospheres, 114(D18), D18119,
doi:10.1029/2009JD011747.
Rawlins, M.A., M.C. Serreze, R. Schroeder, X. Zhang, and K.C. McDonald, 2009b:
Diagnosis of the record discharge of Arctic-draining Eurasian rivers in 2007.
Environmental Research Letters, 4(4), 045011, doi:10.1088/1748-9326/4/4/
045011.
Regehr, E.V., N.J. Lunn, S.C. Amstrup, and I. Stirling, 2007: Effects of earlier sea ice
breakup on survival and population size of polar bears in western Hudson Bay.
Journal of Wildlife Management, 71(8), 2673-2683.
Regehr, E.V., C.M. Hunter, H. Caswell, S.C. Amstrup, and I. Stirling, 2010: Survival and
breeding of polar bears in the southern Beaufort Sea in relation to sea ice. The
Journal of Animal Ecology, 79(1), 117-127.
Regular, P.M., G.J. Robertson, W.A. Montevecchi, F. Shuhood, T. Power, D. Ballam, and
J.F. Piatt, 2010: Relative importance of human activities and climate driving
common murre population trends in the Northwest Atlantic. Polar Biology,
33(9), 1215-1226.
Reinert, E.S., I. Aslaksen, I.M.G. Eira, S.D. Mathiesen, H. Reindert, and E.I. Turi, 2009:
Adapting to climate change in Sámi reindeer herding: the nation-state as
problem and solution. In: Adapting to Climate Change: Thresholds, Values,
Governance [Adger, W.N., I. Lorenzoni, and K.L. O’Brien (eds.)]. Cambridge
University Press, Cambridge, UK and New York, NY, USA, pp. 417-432.
Reinert, H., S. Mathiesen, and E. Reinert, 2010: Climate change and pastoral
flexibility: a Norwegian Saami case. In: The Political Economy of Northern
Regional Development – Yearbook 2008 [Winther, G. (ed.)]. TemaNord
2010:521, Nordic Coucil of Ministers, Copenhagen, Denmark, pp. 189-204.
Reist, J.D., F.J. Wrona, T.D. Prowse, J.B. Dempson, M. Power, G. Kock, T.J. Carmichael,
C.D. Sawatzky, H. Lehtonen, and R.F. Tallman, 2006: Effects of climate change
and UV radiation on fisheries for Arctic freshwater and anadromous species.
AMBIO: A Journal of the Human Environment, 35(7), 402-410.
Rennermalm, A., E. Wood, and T. Troy, 2010: Observed changes in pan-arctic cold-
season minimum monthly river discharge. Climate Dynamics, 35(6), 923-939.
Revich, B., 2008: Climate change alters human health in Russia. Studies in Russian
Economic Development, 19(3), 311-317.
Revich, B. and M. Podolnaya, 2011: Thawing of permafrost may disturb historic cattle
burial grounds in East Siberia. Global Health Action, 4, 8482, doi:10.3402/
gha.v4i0.8482.
Revich, B.A. and D.A. Shaposhnikov, 2010: Extreme temperature episodes and
mortality in Yakutsk, East Siberia. Rural and Remote Health, 10(2), 1338,
www.rrh.org.au/articles/subviewnew.asp?ArticleID=1338.
Revich, B.A. and D.A. Shaposhnikov, 2012: Climate change, heat waves, and cold
spells as risk factors for increased mortality in some regions of Russia. Studies
on Russian Economic Development, 23(2), 195-207.
Revich, B., N. Tokarevich, and A.J. Parkinson, 2012: Climate change and zoonotic
infections in the Russian Arctic. International Journal of Circumpolar Health,
71, 18792, doi:10.3402/ijch.v71i0.18792.
Rice, J.C. and S.M. Garcia, 2011: Fisheries, food security, climate change, and
biodiversity: characteristics of the sector and perspectives on emerging issues.
International Council for the Exploration of the Sea (ICES) Journal of Marine
Science, 68(6), 1343-1353.
Roberts, D., W.R. Howard, A.D. Moy, J.L. Roberts, T.W. Trull, S.G. Bray, and R.R.
Hopcroft, 2011: Interannual pteropod variability in sediment traps deployed
above and below the aragonite saturation horizon in the sub-Antarctic Southern
Ocean. Polar Biology 34(11), 1739-1750.
Robertson, C., T.A. Nelson, D.E. Jelinski, M.A. Wulder, and B. Boots, 2009: Spatial-
temporal analysis of species range expansion: the case of the mountain pine
beetle, Dendroctonus ponderosae. Journal of Biogeography, 36(8), 1446-
1458.
Robinson, R., T. Smith, B.J. Kirschhoffer, and C. Rosa, 2011: Polar bear (Ursus maritimus)
cub mortality at a den site in northern Alaska. Polar Biology, 34(1), 139-142.
Rockwell, R.F. and L.J. Gormezano, 2009: The early bear gets the goose: climate
change, polar bears and lesser snow geese in western Hudson Bay. Polar
Biology, 32(4), 539-547.
Rode, K.D., S.C. Amstrup, and E.V. Regehr, 2010a: Reduced body size and cub
recruitment in polar bears associated with sea ice decline. Ecological
Applications, 20(3), 768-782.
Rode, K.D., J.D. Reist, E. Peacock, and I. Stirling, 2010b: Comments in response to
“Estimating the energetic contribution of polar bear (Ursus maritimus) summer
diets to the total energy budget” by Dyck and Kebreab (2009). Journal of
Mammalogy, 91(6), 1517-1523.
Rode, K.D., E. Peacock, M. Taylor, I. Stirling, E. Born, K. Laidre, and Ø. Wiig, 2012: A
tale of two polar bear populations: ice habitat, harvest, and body condition.
Population Ecology, 54(1), 3-18.
Rogers, A., N. Johnston, E. Murphy, and A. Clarke (eds.), 2012: Antarctic Ecosystems:
An Extreme Environment in a Changing World. John Wiley & Sons, Ltd.,
Chichester, UK, 583 pp.
Roturier, S. and M. Roué, 2009: Of forest, snow and lichen: Sámi reindeer herders’
knowledge of winter pastures in northern Sweden. Forest Ecology and
Management, 258(9), 1960-1967.
Royles, J., M.J. Amesbury, P. Convey, H. Griffiths, D.A. Hodgson, M.J. Leng, and D.J.
Charman, 2013: Plants and soil microbes respond to recent warming on the
Antarctic Peninsula. Current Biology, 23(17), 1702-1706.
Rudberg, P.M., O. Wallgren, and A.G. Swartling, 2012: Beyond generic adaptive
capacity: exploring the adaptation space of the water supply and wastewater
sector of the Stockholm region, Sweden. Climatic Change, 114(3-4), 707-
721.
Rundqvist, S., H. Hedenås, A. Sandström, U. Emanuelsson, H. Eriksson, C. Jonasson,
and T.V. Callaghan, 2011: Tree and shrub expansion over the past 34 years at
the tree-line near Abisko, Sweden. AMBIO: A Journal of the Human Environment,
40(6), 683-692.
Russell, D. and A. Gunn, 2010: Reindeer herding. In: Arctic Biodiversity Trends 2010:
Selected Indicators of Change [Kurvits, T., B. Alfthan, and E. Mork (eds.)].
Conservation of Arctic Flora and Fauna (CAFF) International Secretariat,
Akureyri, Iceland, pp. 86-88.
Rybråten, S. and G. Hovelsrud, 2010: Local effects of global climate change:
differential experiences of sheep farmers and reindeer herders in Unjárga/
Nesseby, a coastal Sámi community in northern Norway. In: Community
Adaptation and Vulnerability in Arctic Regions [Hovelsrud, G.K. and B. Smit
(eds.)]. Springer, Dordrecht, Netherlands, pp. 313-333.

1609
Polar Regions Chapter 28
28
Rylander, R. and R.S.F. Schilling, 2011: Diseases caused by organic dusts. In: 10:
Respiratory System [David, A. and G.R. Wagner (eds.)]. In: Encyclopedia of
Occupational Health and Safety [Stellman, J.M. (Editor-in-Chief)]. International
Labor Organization (ILO), Geneva, Switzerland, 7 pp.
Saba, G.K., O. Schofield, J.J. Torres, E.H. Ombres, and D.K. Steinberg, 2012: Increased
feeding and nutrient excretion of adult Antarctic krill, Euphausia superba,
exposed to enhanced carbon dioxide (CO
2
). PLoS ONE, 7(12), e52224,
doi:10.1371/journal.pone.0052224.
Sahanatien, V. and A.E. Derocher, 2012: Monitoring sea ice habitat fragmentation
for polar bear conservation. Animal Conservation, 15(4), 397-406.
Salick, J. and N. Ross, 2009: Traditional peoples and climate change. Global
Environmental Change, 19(2), 137-139.
Salonen, J.S., H. Seppä, M. Väliranta, V.J. Jones, A. Self, M. Heikkilä, S. Kultti, and H.
Yang, 2011: The Holocene thermal maximum and late-Holocene cooling in the
tundra of NE European Russia. Quaternary Research, 75(3), 501-511.
Schliebe, S., K.D. Rode, J.S. Gleason, J. Wilder, K. Proffitt, T.J. Evans, and S. Miller, 2008:
Effects of sea ice extent and food availability on spatial and temporal distribution
of polar bears during the fall open-water period in the southern Beaufort Sea.
Polar Biology, 31(8), 999-1010.
Schmidt, K., A. Atkinson, S. Steigenberger, S. Fielding, M. Lindsay, D. Pond, G. Tarling,
T. Klevjer, C. Allen, S. Nicol, and E. Achterberg, 2011: Seabed foraging by Antarctic
krill: implications for stock assessment, bentho-pelagic coupling, and the
vertical transfer of iron. Limnology and Oceanography, 56(4), 1411-1428.
Schneider, P. and S.J. Hook, 2010: Space observations of inland water bodies show
rapid surface warming since 1985. Geophysical Research Letters, 37(22),
L22405, doi:10.1029/2010GL045059.
Shaposhnikov, D., B. Revich, V. Meleshko, V. Govorkova, and T. Pavlova, 2010: Climate
change may reduce annual temperature-dependent mortality in Subarctic: a
case study of Archangelsk, Russian Federation. Environment and Natural
Resources Research, 1(1), 75-91.
Shearer, C., 2011: Kivalina: A Climate Change Story. Haymarket Books, Chicago, IL,
USA, 240 pp.
Shearer, C., 2012: The political ecology of climate adaptation assistance: Alaska
Natives, displacement, and relocation. Journal of Political Ecology, 19, 174-183.
Sherman, K., I.M. Belkin, K.D. Friedland, J. O’Reilly, and K. Hyde, 2009: Accelerated
warming and emergent trends in fisheries biomass yields of the world’s large
marine ecosystems. AMBIO: Journal of the Human Environment, 38(4), 215-224.
Shiklomanov, A.I., R.B. Lammers, M.A. Rawlins, L.C. Smith, and T.M. Pavelsky, 2007:
Temporal and spatial variations in maximum river discharge from a new
Russian data set. Journal of Geophysical Research: Biogeosciences, 112(G4),
G04S53, doi:10.1029/2006JG000352.
Shiyatov, S.G., M.M. Terent’ev, V.V. Fomin, and N.E. Zimmermann, 2007: Altitudinal
and horizontal shifts of the upper boundaries of open and closed forests in the
Polar Urals in the 20
th
century. Russian Journal of Ecology, 38(4), 223-227.
Shreeve, R.S., M.A. Collins, G.A. Tarling, C.E. Main, P. Ward, and N.M. Johnston, 2009:
Feeding ecology of myctophid fishes in the northern Scotia Sea. Marine Ecology
Progress Series, 386, 221-236.
Sigler, M.F., M. Renner, S.L. Danielson, L.B. Eisner, R.R. Lauth, K.J. Kuletz, E.A.
Logerwell, and G.L. Hunt Jr., 2011: Fluxes, fins, and feathers: relationships
among the Bering, Chukchi, and Beaufort Seas in a time of climate change.
Oceanography, 24(3), 250-265.
Sigurdsson, B.D., A. Snorrason, B.T. Kjartansson, and J.A. Jonsson, 2007: Total area
of planted forests in Iceland and their carbon stocks and fluxes. In: Effects of
Afforestation on Ecosystems, Landscape and Rural Development [Halldórsson,
G., E.S. Oddsdóttir, andO. Eggertsson (eds.)]. TemaNord 2007:508, Proceedings
of the AFFORNORD Conference, Reykholt, Iceland, June 18-22, 2005, Nordic
Council of Ministers, Copenhagen, Denmark, pp. 211-217.
Simpson, S., S. Jennings, M. Johnson, J. Blanchard, P.-J. Schön, D. Sims, and M. Genner,
2011: Continental shelf-wide response of a fish assemblage to rapid warming
of the sea. Current Biology, 21(18), 1565-1570.
Siniff, D.B., R.A. Garrott, J.J. Rotella, W.R. Fraser, and D.G. Ainley, 2008: Opinion:
projecting the effects of environmental change on Antarctic seals. Antarctic
Science, 20(5), 425-435.
Slagstad, D., I.H. Ellingsen, and P. Wassmann, 2011: Evaluating primary and secondary
production in an Arctic Ocean void of summer sea ice: an experimental
simulation approach. Progress in Oceanography, 90(1-4), 117-131.
Slater, G.J., B. Figueirido, L. Louis, P. Yang, and B. van Valkenburgh, 2010: Biomechanical
consequences of rapid evolution in the polar bear lineage. PLoS ONE, 5(11),
e13870, doi:10.1371/journal.pone.0013870.
Smith, J.A., D.A. Hodgson, M.J. Bentley, E. Verleyen, M.J. Leng, and S.J. Roberts, 2006:
Limnology of two Antarctic epishelf lakes and their potential to record periods
of ice shelf loss. Journal of Paleolimnology, 35(2), 373-394.
Smith, L.C., T.M. Pavelsky, G.M. MacDonald, A.I. Shiklomanov, and R.B. Lammers, 2007:
Rising minimum daily flows in northern Eurasian rivers: a growing influence of
groundwater in the high-latitude hydrologic cycle. Journal of Geophysical
Research: Biogeosciences, 112(G4), G04S47, doi:10.1029/2006JG000327.
Smith, P.A., K.H. Elliott, A.J. Gaston, and H.G. Gilchrist, 2010: Has early ice clearance
increased predation on breeding birds by polar bears? Polar Biology, 33(8),
1149-1153.
Smith, S.L. and D.W. Riseborough, 2010: Modelling the thermal response of permafrost
terrain to right-of-way disturbance and climate warming.Cold Regions Science
and Technology, 60(1), 92-103.
Smith Jr., W., D. Ainley, R. Cattaneo-Vietti, and E. Hofmann, 2012: The Ross Sea
continental shelf: regional biogeochemical cycles, trophic interactions, and
potential future changes. In: Antarctic Ecosystems: An Extreme Environment in
a Changing World [Rogers, A., N. Johnston, E. Murphy, and A. Clarke (eds.)].
Blackwell Publishing, London, UK, pp. 213-242.
Smol, J.P. and M.S.V. Douglas, 2007a: Crossing the final ecological threshold in High
Arctic ponds. Proceedings of the National Academy of Sciences of the United
States of America, 104(30), 12395-12397.
Smol, J.P. and M.S.V. Douglas, 2007b: From controversy to consensus: making the
case for recent climate change in the Arctic using lake sediments. Frontiers in
Ecology and the Environment, 5(9), 466-474.
Søreide, J.E., E. Leu, J. Berge, M. Graeve, and S. Falk-Petersen, 2010: Timing of blooms,
algal food quality and Calanus glacialis reproduction and growth in a changing
Arctic. Global Change Biology, 16(11), 3154-3163.
St. Jacques, J. and D.J. Sauchyn, 2009: Increasing winter baseflow and mean annual
streamflow from possible permafrost thawing in the Northwest Territories, Canada.
Geophysical Research Letters, 36(1), L01401, doi:10.1029/2008GL035822.
Stabeno, P.J., N.B. Kachel, S.E. Moore, J.M. Napp, M. Sigler, A. Yamaguchi, and A.N.
Zerbini, 2012a: Comparison of warm and cold years on the southeastern Bering
Sea shelf and some implications for the ecosystem. Deep-Sea Research Part II:
Topical Studies in Oceanography, 65-70, 31-45.
Stabeno, P.J., E.V. Farley Jr., N.B. Kachel, S. Moore, C.W. Mordy, J.M. Napp, J.E.
Overland, A.I. Pinchuk, and M.F. Sigler, 2012b: Comparison of the physics of
the northern and southern shelves of the eastern Bering Sea and some
implications for the ecosystem. Deep-Sea Research Part II: Topical Studies in
Oceanography, 65-70, 14-30.
Stammler, F., 2005: Reindeer Nomads Meet the Market: Culture, Property and
Globalisation at the End of the Land. LIT Verlag, Berlin, Germany and Transaction
Publishers, Piscataway, NJ, USA, 379 pp.
Stephenson, S.R., L.C. Smith, and J.A. Agnew, 2011: Divergent long-term trajectories
of human access to the Arctic. Nature Climate Change, 1(3), 156-160.
Stewart, E.J., S.E.L. Howell, D. Draper, J. Yackel, and A. Tivy, 2007: Sea ice in Canada’s
Arctic: implications for cruise tourism. Artic, 60(4), 370-380.
Stewart, E.J., S.E.L. Howell, J.D. Dawson, A. Tivy, and D. Draper, 2010: Cruise tourism
and sea ice in Canada’s Hudson Bay region. Artic, 63, 57-66.
Stien, A., L.E. Loel, A. Mysterud, T. Severinsen, J. Kohler, and R. Langvatn, 2010: Icing
events trigger range displacement in a high-arctic ungulate. Ecology, 91(3),
915-920.
Stien, A., R.A. Ims, S.D. Albon, E. Fuglei, R.J. Irvine, E. Ropstad, O. Halvorsen, R. Langvatn,
L.E. Loe, V. Veiberg, and N.G. Yoccoz, 2012: Congruent responses to weather
variability in high Arctic herbivores. Biology Letters, 8(6), 1002-1005.
Stirling, I. and C.L. Parkinson, 2006: Possible effects of climate warming on selected
populations of polar bears (Ursus maritimus) in the Canadian Arctic. Arctic,
59(3), 261-275.
Stirling, I., E. Richardson, G.W. Thiemann, and A.E. Derocher, 2008a: Unusual predation
attempts of polar bears on ringed seals in the southern Beaufort Sea: possible
significance of changing spring ice conditions. Arctic, 61(1), 14-22.
Stirling, I., A.E. Derocher, W.A. Gough, and K. Rode, 2008b: Response to Dyck et al.
(2007) on polar bears and climate change in western Hudson Bay. Ecological
Complexity, 5(3), 193-201.
Stirling, I., T.L. McDonald, E.S. Richardson, E.V. Regehr, and S.C. Amstrup, 2011: Polar
bear population status in the northern Beaufort Sea, Canada, 1971-2006.
Ecological Applications, 21(3), 859-876.
Stram, D.L. and D.C.K. Evans, 2009: Fishery management responses to climate change
in the North Pacific. International Council for the Exploration of the Sea (ICES)
Journal of Marine Science, 66(7), 1633-1639.

1610
Chapter 28 Polar Regions
28
Stroeve, J., M.M. Holland, W. Meier, T. Scambos, and M. Serreze, 2007: Arctic sea ice
decline: faster than forecast. Geophysical Research Letters, 34(9), L09501,
doi:10.1029/2007GL029703.
Sundby, S. and O. Nakken, 2008: Spatial shifts in spawning habitats of Arcto-
Norwegian cod related to multidecadal climate oscillations and climate change.
International Journal for the Exploration of the Sea (ICES) Journal of Marine
Science, 65(6), 953-962.
Swann, A.L., I.Y. Fung, S. Levis, G. Bonan, and S. Doney, 2010: Changes in Arctic
vegetation induce high-latitude warming through the greenhouse effect.
Proceedings of the National Academy of Sciences of the United States of
America, 107(4), 1295-1300.
Sydneysmith, R., M. Andrachuk, B. Smit, and G.K. Hovelsrud, 2010: Vulnerability and
adaptive capacity in Arctic communities. In: Adaptive Capacity and Environmental
Governance [Armitage, D. and R. Plummer (eds.)]. Springer, Berlin, Germany, pp.
133-156.
Tan, A., J.C. Adam, and D.P. Lettenmaier, 2011: Change in spring snowmelt timing in
Eurasian Arctic rivers. Journal of Geophysical Research: Atmospheres, 116(D3),
D03101, doi:10.1029/2010JD014337.
Tanabe, Y., S. Kudoh, S. Imura, and M. Fukuchi, 2007: Phytoplankton blooms under
dim and cold conditions in freshwater lakes of East Antarctica. Polar Biology,
31(2), 199-208.
Tchebakova, N.M., G.E. Rehfeldt, and E.I. Parfenova, 2009: From vegetation zones
to climatypes: effects of climate warming on Siberian ecosystems. In: Permafrost
Ecosystems: Siberian Larch Forest [Osawa, A., O.A. Zyryanova, Y. Matsuura, T.
Kajimoto, and R.W. Wein (eds.)] Ecological Studies, Vol. 109, Springer, Dordrecht,
Netherlands, pp. 427-446.
Teng, Y., Z. Xu, Y. Luo, and F. Reverchon, 2012: How do persistent organic pollutants
be coupled with biogeochemical cycles of carbon and nutrients in terrestrial
ecosystems under global climate change? Journal of Soils and Sediments,
12(3), 411-419.
Teran, T., L. Lamon, and A. Marcomini, 2012: Climate change effects on POPs’
environmental behavior: a scientific perspective for future regulatory actions.
Atmospheric Pollution Research, 3(4), 466-476.
Terauds, A., S.L. Chown, F. Morgan, H. J. Peat, D.J. Watts, H. Keys, P. Convey, and D.M.
Bergstrom, 2012: Conservation biogeography of the Antarctic. Diversity and
Distributions, 18(7), 726-741.
Thiemann, G.W., S.J. Iverson, and I. Stirling, 2008: Polar bear diets and arctic marine
food webs: insights from fatty acid analysis. Ecological Monographs, 78(4),
591-613.
Tholstrup, L. and R.O. Rasmussen (eds.), 2009: Climate Change and the North
Atlantic. Nordic Atlantic Cooperation (NORA), Tórshavn, Faroe Islands, 130 pp.
Thompson, M., F. Wrona, and T. Prowse, 2012: Shifts in plankton, nutrient and light
relationships in small tundra lakes caused by localized permafrost thaw. Arctic,
65(4), 367-376.
Thompson, M.S., S.V. Kokelj, T.D. Prowse, and F.J. Wrona, 2008: The impact of
sediments derived from thawing permafrost on tundra lake water chemistry:
an experimental approach. In: Proceedings of the Ninth International Conference
on Permafrost [D.L. Kane and K.M. Hinkel (eds.)]. Institute of Northern Engineering,
University of Alaska, Fairbanks, AK, USA, pp. 1763-1768.
Thrush, S.F. and V.J. Cummings, 2011: Massive icebergs, alteration in primary food
resources and change in benthic communities at Cape Evans, Antarctica. Marine
Ecology: An Evolutionary Perspective, 32(3), 289-299.
Tokarevich, N., A. Tronin, O. Blinova, R. Buzinov, V. Boltenkov, E. Yurasova, and J.
Nurse, 2011: The impact of climate change on the expansion of Ixodes
persulcatus habitat and the incidence of tickborne encephalitis in the north
of European Russia. Global Health Action, 4, 8448, doi:10.3402/gha.
v4i0.8448.
Tømmervik, H., B. Johansen, J.A. Riseth, S.R. Karlsen, B. Solberg, and K.A. Høgda,
2009: Above ground biomass changes in the mountain birch forests and
mountain heaths of Finnmarksvidda, northern Norway, in the period 1957-
2006. Forest Ecology and Management, 257(1), 244-257.
Tømmervik, H., J.W. Bjerke, E. Gaare, B. Johansen, and D. Thannheiser, 2012: Rapid
recovery of recently overexploited winter grazing pastures for reindeer in northern
Norway. Fungal Ecology, 5(1), 3-15.
Towns, L., A.E. Derocher, I. Stirling, and N.J. Lunn, 2010: Changes in land distribution
of polar bears in western Hudson Bay. Arctic, 63(2), 206-212.
Towns, L., A.E. Derocher, I. Stirling, N.J. Lunn, and D. Hedman, 2009: Spatial and
temporal patterns of problem polar bears in Churchill, Manitoba. Polar Biology,
32(10), 1529-1537.
Trathan, P.N. and D. Agnew, 2010: Climate change and the Antarctic marine
ecosystem: an essay on management implications. Antarctic Science, 22(4),
387-398.
Trathan, P.N. and K. Reid, 2009: Exploitation of the marine ecosystem in the sub-
Antarctic: historical impacts and current consequences. Papers and Proceeding
of the Royal Society of Tasmania, 143(1), 9-14.
Trathan, P.N., J. Forcada, and E.J. Murphy, 2007: Environmental forcing and Southern
Ocean marine predator populations: effects of climate change and variability.
Philosophical Transactions of the Royal Society B, 362(1488), 2351-2365.
Trathan, P., P. Fretwell, and B. Stonehouse, 2011: First recorded loss of an emperor
penguin colony in the recent period of Antarctic regional warming: implications
for other colonies. PLoS ONE, 6(2), e14738, doi:10.1371/journal.pone.0014738.
Trathan, P.N., J. Forcada, and E.J. Murphy, 2012: Environmental forcing and Southern
Ocean marine predator populations. In: Antarctic Ecosystems: An Extreme
Environment in a Changing World [Rogers, A., N. Johnston, E. Murphy, and A.
Clarke (eds.)]. John Wiley & Sons, Chichester, UK, pp. 335-354.
Tremblay, J., D. Robert, D.E. Varela, C. Lovejoy, G. Darnis, R.J. Nelson, and A.R. Sastri,
2012: Current state and trends in Canadian Arctic marine ecosystems: I. Primary
production. Climatic Change, 115(1), 161-178.
Trivelpiece, W.Z., J.T. Hinke, A.K. Miller, C.S. Reiss, S.G. Trivelpiece, and G.M. Watters,
2011: Variability in krill biomass links harvesting and climate warming to
penguin population changes in Antarctica. Proceedings of the National
Academy of Sciences of the United States of America, 108(18), 7625-7628.
Turner, J., R.A. Bindschadler, P. Convey, G. Di Prisco, E. Fahrbach, J. Gutt, D.A. Hodgson,
P.A. Mayewski, and C.P. Summerhayes (eds.), 2009: Antarctic Climate Change
and the Environment. Scientific Committee on Antarctic Research (SCAR),
Cambridge, UK, 526 pp.
Turner, J.A., N.E. Barrand, T.J. Bracegirdle, P. Convey, D.A. Hodgson, M. Jarvis, A. Jenkins,
G. Marshall, M.P. Meredith, H. Roscos, J. Shanklin, J. French, H. Goosse, M.
Guglielmin, J. Gutt, S. Jacobs, M.C. Kennicutt II, V. Masson-Delmotte, P.
Mayewski, F. Navarro, S. Robinson, T. Scambos, M. Sparrow, C. Summerhayes,
K. Speer, and A. Klepnikov, 2013: Antarctic climate change and the environment:
an update. Polar Record (in press), doi:10.1017/S0032247413000296.
Turunen, M., P. Soppela, H. Kinnunen, M.-L. Sutinen, and F. Martz, 2009: Does climate
change influence the availability and quality of reindeer forage plants? Polar
Biology, 32(6), 813-832.
Tyler, N.J.C., 2010: Climate, snow, ice, crashes, and declines in populations of reindeer
and caribou (Rangifer tarandus L.). Ecological Monographs, 80(2), 197-219.
Tyler, N.J.C., J.M. Turi, M.A. Sundset, K. Strøm Bull, M.N. Sara, E. Reinert, N. Oskal, C.
Nellemann, J.J. McCarthy, S.D. Mathiesen, M.L. Martello, O.H. Magga, G.K.
Hovelsrud, I. Hanssen-Bauer, N.I. Eira, I.M.G. Eira, and R.W. Corell, 2007: Saami
reindeer pastoralism under climate change: applying a generalized framework
for vulnerability studies to a sub-Arctic social-ecological system. Global
Environmental Change: Human and Policy Dimensions, 17(2), 191-206.
Tyler, N.J.C., M.C. Forchhammer, and N.A. Øritsland, 2008: Nonlinear effects of
climate and density in the dynamics of a fluctuating population of reindeer.
Ecology, 89(6), 1675-1686.
UNEP and AMAP, 2011: Climate Change and POPs: Predicting the Impacts. Report
of the United Nations Environment Programne (UNEP) and Arctic Monitoring
and Assessment Programme (AMAP) Expert Group, Secretariat of the Stockholm
Convention, Geneva, Switzerland, 62 pp.
USARC, 2010: Report on Goals and Objectives for Arctic Research 2009-2010. United
States Arctic Research Commission (USARC), Arlington, VA, USA, 52 pp.
Valdimarsson, H., O.S. Astthorsson, and J. Palsson, 2012: Hydrographic variability in
Icelandic waters during recent decades and related changes in distribution of
some fish species. International Council for the Exploration of the Sea (ICES)
Journal of Marine Science, 69(5), 815-825.
Valdivia, C., A. Seth, J.L. Gilles, M. García, E. Jiménez, J. Cusicanqui, F. Navia, and E.
Yucra, 2010: Adapting to climate change in Andean ecosystems: landscapes,
capitals, perceptions shaping rural livelihood strategies and linking knowledge
systems. Annals of the Association of American Geographers, 100(4), 818-
834.
Van Bogaert, R., C. Jonasson, M. De Dapper, and T.V. Callaghan, 2010: Range
expansion of thermophilic aspen (Populus tremula L.) in the Swedish subarctic.
Arctic, Antarctic, and Alpine Research, 42(3), 362-375.
Van Bogaert, R., K. Haneca, J. Hoogesteger, C. Jonasson, M. De Dapper, and T.V.
Callaghan, 2011: A century of tree line changes in sub-Arctic Sweden shows
local and regional variability and only a minor influence of 20
th
century climate
warming. Journal of Biogeography, 38(5), 907-921.

1611
Polar Regions Chapter 28
28
van Der Wal, R., S. Sjögersten, S.J. Woodin, E.J. Cooper, I.S. Jónsdóttir, D. Kuijper, T.A.D.
Fox, and A.D. Huiskes, 2007: Spring feeding by pink-footed geese reduces
carbon stocks and sink strength in tundra ecosystems. Global Change Biology,
13(2), 539-545.
Veillette, J., D.R. Mueller, D. Antoniades, and W.F. Vincent, 2008: Arctic epishelf lakes
as sentinel ecosystems: past, present and future. Journal of Geophysical
Research: Biogeosciences, 113(G4), G04014, doi:10.1029/2008JG000730.
Velázquez, D., A. Frias, M.A. Lezcano, and A. Quesada, 2013: Ecological relationships
and stoichiometry within a maritime Antarctic watershed. Antarctic Science,
25(2), 191-197.
Verbyla, D., 2008: The greening and browning of Alaska based on 1982-2003 satellite
data. Global Ecology and Biogeography, 17(4), 547-555.
Vert-pre, K.A., R.O. Amoroso, O.P. Jensen, and R. Hilborn, 2013: Frequency and intensity
of productivity regime shifts in marine stocks. Proceedings of the National
Academy of Sciences of the United States of America, 110(5), 1779-1784.
Vieira, G., J. Bockheim, M. Guglielmin, M. Balks, A.A. Abramov, J. Boelhouwers, N.
Cannone, L. Ganzert, D.A. Gilichinsky, S. Goryachkin, J. López-Martínez, I.
Meiklejohn, R. Raffi, M. Ramos, C. Schaefer, E. Serrano, F. Simas, R. Sletten, and
D. Wagner, 2010: Thermal state of permafrost and active-layer monitoring in
the Antarctic: advances during the International Polar Year 2007-09. Permafrost
and Periglacial Processes, 21(2), 182-197.
Vikebø, F.B., Å. Huseø, A. Slotte, E.K. Stenevik, and V.S. Lien, 2010: Effect of hatching
date, vertical distribution and interannual variation in physical forcing on
northward displacement and temperature conditions of Norwegian spring-
spawning herring larvae. International Council for the Exploration of the Sea
(ICES) Journal of Marine Science, 67(9), 1948-1956.
Vikhamar-Schuler, D., I. Hanssen-Bauer, T.V. Schuler, S.D. Mathiesen, and M. Lehning,
2013: Use of a multilayer snow model to assess grazing conditions for reindeer.
Annals of Glaciology, 54(62), 214-226.
Vincent, W., J. Hobbie, J. Laybourn-Parry, 2008: Introduction to the limnology of high-
latitude lake and river ecosystems. In: Polar Lakes and Rivers: Limnology of
Arctic and Antarctic Aquatic Ecosystems [Vincent, W.F. and J. Laybourn-Parry
(eds.)]. Oxford University Press, Oxford, UK, pp. 1-18.
Vincent, W.F., L.G. Whyte, C. Lovejoy, C.W. Greer, I. Laurion, C.A. Suttle, J. Corbeil,
and D.R. Mueller, 2009: Arctic microbial ecosystems and impacts of extreme
warming during the International Polar Year. Polar Science, 3(3), 171-180.
Virginia, R.S. and K.S. Yalowitz (eds.), 2012: A New Paradigm for Arctic Health:
Challenges and Responses to Rapid Climate, Environmental, and Social Change.
Report on the International Workshop, “Arctic Health: Challenges and
Responses to Rapid Climate, Environmental, and Social Change,” May 23-24,
2011, Organized by the Dickey Center for International Understanding,
Dartmouth College, Hanover, NH and the University of the Arctic Institute for
Applied Circumpolar Policy, Dickey Center Institute of Arctic Studies Policy Paper
No. 2, Darmouth College, Hanover, NH, USA, 17 pp., http://iacp.dartmouth.edu/
images/stories/2012_Health_Report.pdf.
Voggesser, G., K. Lynn, J. Daigle, F. Lake, and D. Ranco, 2013: Cultural impacts to tribes
from climate change influences on forests. Climatic Change, 12(3), 615-626.
von Biela, V.R., C.E. Zimmerman, and L.L. Moulton, 2011: Long-term increases in young-
of-the-year growth of Arctic cisco Coregonus autumnalis and environmental
influences. Journal Fish Biology, 78(1), 39-56.
Vors, L.S. and M.S. Boyce, 2009: Global declines of caribou and reindeer. Global
Change Biology, 15(11), 2626-2633.
Vuojala-Magga, T., M. Turunen, T. Ryyppö, and M. Tennberg, 2011: Resonance strategies
of Sámi reindeer herders in northernmost Finland during climatically extreme
years. Arctic, 64(2), 227-241.
Vyverman, W., E. Verleyen, A. Wilmotte, D.A. Hodgson, A. Willems, K. Peeters, B. Van
de Vijver, A. De Wever, F. Leliaert, and K. Sabbe, 2010: Evidence for widespread
endemism among Antarctic micro-organisms. Polar Science, 4(2), 103-113.
Walker, D.A., M.O. Leibman, H.E. Epstein, B.C. Forbes, U.S. Bhatt, M.K. Raynolds, J.C.
Comiso, A.A. Gubarkov, A.V. Khomutov, G.J. Jia, E. Kaarlejärvi, J.O. Kaplan, T.
Kumpula, P. Kuss, G. Matyshak, N.G. Moskalenko, P. Orekhov, V.E. Romanovsky,
N.G. Ukraientseva, and Q. Yu, 2009: Spatial and temporal patterns of greenness
on the Yamal Peninsula, Russia: interactions of ecological and social factors
affecting the Arctic normalized difference vegetation index. Environmental
Research Letters, 4(4), 045004,doi:10.1088/1748-9326/4/4/045004.
Walker, D.A., P. Kuss, H.E. Epstein, A.N. Kade, C.M. Vonlanthen, M.K. Raynolds, and
F.J. Daniëls, 2011: Vegetation of zonal patterned-ground ecosystems along the
North America Arctic bioclimate gradient. Applied Vegetation Science, 14(4),
440-463.
Walsh, J.J., D.A. Dieterle, F.R.Chen, J.M. Lenes, W. Maslowski, J.J. Cassano, T.E. Whitledge,
D. Stockwell, M. Flint, R.N. Sukhanova, and J. Christensen, 2011: Trophic cascades
and future harmful algal blooms within ice-free Arctic seas north of Bering Strait:
a simulation analysis. Progress in Oceanography, 91(3), 312-343.
Walter, K.M., L.C. Smith, and F.S. Chapin III, 2007a: Methane bubbling from northern
lakes: present and future contributions to the global methane budget.
Philosophical Transactions of the Royal Society A, 365(1856), 1657-1676.
Walter, K.M., M.E. Edwards, G. Grosse, S.A. Zimov, and F.S. Chapin III, 2007b:
Thermokarst lakes as a source of atmospheric CH
4
during the last deglaciation.
Science, 318(5850), 633-636.
Walter, K.M., J.P. Chanton, F.S. Chapin III, E.A.G. Schuur, and S.A. Zimov, 2008: Methane
production and bubble emissions from arctic lakes: isotopic implications for
source pathways and ages. Journal of Geophysical Research: Biogeosciences,
113(G3), G00A08, doi:10.1029/2007JG000569.
Waluda, C.M., M.A. Collins, A.D. Black, I.J. Staniland, and P.N. Trathan, 2010: Linking
predator and prey behaviour: contrasts between Antarctic fur seals and
macaroni penguins at South Georgia. Marine Biology, 157(1), 99-112.
Walvoord, M.A. and R.G. Striegl, 2007: Increased groundwater to stream discharge
from permafrost thawing in the Yukon River basin: potential impacts on lateral
export of carbon and nitrogen. Geophysical Research Letters, 34(12), L12402,
doi:10.1029/2007GL030216.
Wang, M., and J.E. Overland, 2009: A sea ice free summer Arctic within 30 years?
Geophysical Research Letters, 36(7), L07502, doi:10.1029/2009GL037820.
Wasley, J., S.A. Robinson, M. Popp, and C.E. Lovelock, 2006: Some like it wet biological
characteristics underpinning tolerance of extreme water events in Antarctic
bryophytes. Functional Plant Biology, 33, 443-455.
Wassmann, P., 2011: Arctic marine ecosystems in an era of rapid climate change.
Progress in Oceanography, 90(1-4), 1-17.
Wassman, P., C. M. Duarte, S. Agusti, and M. K. Sejr, 2011: Footprints of climate
change in the Arctic marine ecosystem. Global Change Biology, 17, 1235-1249.
Watson, S., L.S. Peck, P.A. Tyler, P.C. Southgate, K.S. Tan, R.W. Day, and S.A. Morley,
2012: Marine invertebrate skeleton size varies with latitude, temperature and
carbonate saturation: implications for global change and ocean acidification.
Global Change Biology, 18(10), 3026-3038.
Weatherhead, E., S. Gearheard, and R.G. Barry, 2010: Changes in weather persistence:
insight from Inuit knowledge. Global Environmental Change, 20(3), 523-528.
Weimerskirch, H., P. Inchausti, C. Guinet, and C. Barbraud, 2003: Trends in bird and
seal populations as indicators of a system shift in the Southern Ocean. Antarctic
Science, 15(2), 249-256.
Weimerskirch, H., M. Louzao, S. de Grissac, and K. Delord, 2012: Changes in wind
pattern alter albatross distribution and life-history traits. Science, 335(6065),
211-214.
Wenzel, G.W., 2009: Canadian Inuit subsistence and ecological instability? If the
climate changes, must the Inuit? Polar Research, 28(1), 89-99.
West, J. and G. Hovelsrud, 2010: Cross-scale adaptation challenges in the coastal
fisheries: findings from Lebesby, northern Norway. Arctic, 63(3), 338-354.
Whyte, K.P. 2013: Justice forward: tribes, climate adaptation and responsibility.
Climatic Change, 12(3 SI), 517-530.
Wiedenmann, J., K. Cresswell, and M. Mangel, 2008: Temperature-dependent growth
of Antarctic krill: predictions for a changing climate from a cohort model.
Marine Ecology Progress Series, 358, 191-202.
Wiedenmann, J., K.A. Cresswell, and M. Mangel, 2009: Connecting recruitment of
Antarctic krill and sea ice. Limonology and Oceanography, 54(3), 799-811.
Wiig, Ø., J. Aars, and E.W. Born, 2008: Effects of climate change on polar bears.
Science Progress, 91(Pt 2), 151-173.
Wildcat, D., 2009: Red Alert! Saving the Planet with Indigenous Knowledge. Fulcrum
Publishing, Golden, CO, USA, 143 pp.
Wilderbuer, T., W. Stockhausen, and N. Bond, 2013: Updated analysis of flatfish
recruitment response to climate variability and ocean conditions in the eastern
Bering Sea. Deep-Sea Research II: Topical Studies in Oceanography, 94, 157-
164.
Williams, T. and P. Hardison, 2013: Culture, law, risk and governance: contexts of
traditional knowledge in climate change adaptation. Climatic Change, 12(3),
531-544.
Williams, T.M., S.R. Noren, and M. Glenn, 2011: Extreme physiological adaptations as
predictors of climate-change sensitivity in the narwhal, Monodon monoceros.
Marine Mammal Science, 27, 334-349.
Wilson, W.J. and O.A.Ormseth, 2009: A new management plan for Arctic waters of
the United States. Fisheries, 34(11), 555-558.

1612
Chapter 28 Polar Regions
28
Wolf, A., T.V. Callaghan, and K. Larson, 2008: Future changes in vegetation and
ecosystem function of the Barents Region. Climatic Change, 87(1-2), 51-73.
Wramneby, A., B. Smith, and P. Samuelsson, 2010: Hot spots of vegetation – climate
feedbacks under future greenhouse forcing in Europe. Journal of Geophysical
Research D: Atmospheres, 115(D21), D21119, doi:10.1029/2010JD014307.
Xu, L., R.B. Myneni, F.S. Chapin III, T.V. Callaghan, J.E. Pinzon, C.J. Tucker, Z. Zhu, J. Bi,
P. Ciais, H. Tommervik, E.S. Euskirchen, B.C. Forbes, S.L. Piao, B.T. Anderson, S.
Ganguly, R.R. Nemani, S.J. Goetz, P.S.A. Beck, A.G. Bunn, C. Cao, and J.C. Stroeve,
2013: Temperature and vegetation seasonality diminishment over northern
lands. Nature Climate Change, 3, 581-586.
Yamamoto-Kawai, M., F.A. McLaughlin, E.C. Carmack, S. Nishino, and K. Shimada,
2009: Aragonite undersaturation in the Arctic Ocean: effects of ocean
acidification and sea ice melt. Science, 326(5956), 1098-1100.
Yarie, J., 2008: Effects of moisture limitation on tree growth in upland and floodplain
forest ecosystems in interior Alaska. Forest Ecology and Management, 256(5),
1055-1063.
Ye, B., D. Yang, Z. Zhang, and D.L. Kane, 2009: Variation of hydrological regime with
permafrost coverage over Lena Basin in Siberia. Journal of Geophysical
Research: Atmospheres, 114(D7), D07102, doi:10.1029/2008JD010537.
Zarnetske, J.P., M.N. Gooseff, W.B. Bowden, M.J. Greenwald, T.R. Brosten, J.H.
Bradford, and J.P. McNamara, 2008: Influence of morphology and permafrost
dynamics on hyporheic exchange in Arctic headwater streams under warming
climate conditions. Geophysical Research Letters, 35(2), L02501, doi:10.1029/
2007GL032049.
Zeng, H., G. Jia, and B. Forbes, 2013: Shifts in Arctic phenology in response to climate
and anthropogenic factors as detected from multiple satellite time series.
Environmental Research Letters 8(3), 035036, doi:10.1088/1748-9326/8/3/
035036.
Zerbini, A., P. Clapham, and P. Wade, 2010: Assessing plausible rates of population
growth in humpback whales from life-history data. Marine Biology, 157(6),
1225-1236.
Zhang, J., Y.H. Spitz, M. Steele, C. Ashjian, R. Campbell, L. Berline, and P. Matrai, 2010:
Modeling the impact of declining sea ice on the Arctic marine planktonic
ecosystem. Journal of Geophysical Research: Oceans, 115(C10), C10015,
doi:10.1029/2009JC005387.
Zhang, K., J.S. Kimball, E.H. Hogg, M. Zhao, W.C. Oechel, J.J. Cassano, and S.W.
Running, 2008: Satellite-based model detection of recent climate-driven
changes in northern high-latitude vegetation productivity. Journal of Geophysical
Research: Biogeosciences, 113(G3), G03033, doi:10.1029/2007JG000621.
Zhang, X., J. He, J. Zhang, I. Polyakov, I., R. Gerdes, J. Inoue, and P. Wu, 2013: Enhanced
poleward moisture transport and amplified high-latitude wetting trend. Nature
Climate Change, 3, 47-51.
Zhukova, N.G., V.N. Nesterova, I.P. Prokopchuk, and G.B. Rudneva, 2009: Winter
distribution of euphausiids (Euphausiacea) in the Barents Sea (2000-2005).
Deep-Sea Research Part II: Topical Studies in Oceanography, 56(21-22), 1959-
1967.
